Nitrile to Amide

Common Conditions:
NaOH
The conversion of nitriles to amides by partial hydrolysis with NaOH (or KOH, LiOH, etc.) is usually best acheived by using mild heating and monitoring the reaction carefully. The amide product readily hydrolyzes to the carboxylic acid so high yields can be difficult.[1][2]
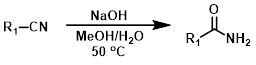
UHP
A mild method for converting nitriles to amides involves using an alkaline solution (ex. NaOH in aq. EtOH) of hydrogen peroxide (H2O2). Urea-Hydrogen Peroxide (UHP) is a solid reagent that upon dissolving provides free H2O2.[1]
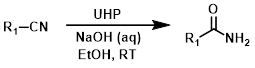
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
 |
|||
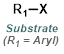 |
||||
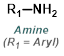 |
References:
1) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
1) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
