Iodine
General Information:
Structure:
![]()
CAS Number: 7553-56-2
Molecular Weight: 253.81 g/mol
Appearance: Metallic gray solid
Melting Point: 113 C
Boiling Point: 184 C
Iodine exists mainly in its diatomic form as I2. It is a metallic gray solid which slowly sublimes to give a purple gas. Iodine is useful as a reagent for various iodination reactions. Iodine is also commonly used as a stain for visualizing compounds on TLC plates.
Common Uses:
Reagent for the iodination of aromatic ring systems
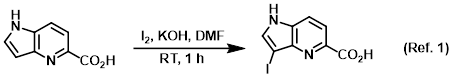
Procedure excerpt:
To a solution of the SM (400 mg, 2.467 mmol) in DMF (4.9 mL) was added Iodine (626 mg, 2.467 mmol) and KOH (346 mg, 6.17 mmol). The mixture was stirred at RT . . .
Reagent for the conversion of alcohols to iodides
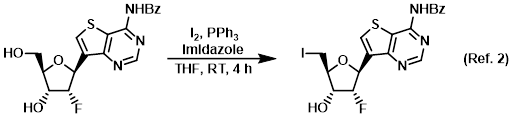
Procedure excerpt:
To a solution of the SM (0.79 g, 2.03 mmol), PPh3 (1.20 g, 4.58 mmol), and imidazole (0.277 g, 4.07 mmol) in THF (15 mL) was added iodine (0.96 g, 3.78 mmol) at RT. . . .
Safety:
Elemental iodine (I2) is toxic if taken orally.
References:
1) Patent Reference: WO2012129338, page 84, ![]() (12.0 MB)
(12.0 MB)
2) Patent Reference: WO2016018697, page 79, ![]() (5.3 MB)
(5.3 MB)
3) Wikipedia: Iodine (link)
4) www.sigmaaldrich.com: Iodine (link)
