Nitrile to Acid

Common Conditions:
NaOH
The basic hydrolysis of a nitrile to a carboxylic acid is typically done with a hydroxide base (ex. NaOH or KOH) in an appropriate solvent (ex. EtOH/H2O) at high temperatures (ex. reflux). Base sensitive substrates may not be well tolerated.[1][2]
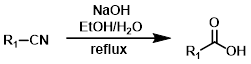
HCl
The acidic hydrolysis of a nitrile to a carboxylic acid is typically done with a strong acid (ex. HCl) at high temperatures (ex. reflux). Acid sensitive substrates may not be well tolerated.[1][2]
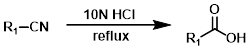
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
 |
|||
 |
 |
|||
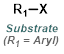 |
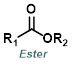 |
|||
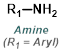 |
 |
|||
 |
References:
1) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
2) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
