Nitrile to Acid
(Basic Conditions)
Examples:
Example 1
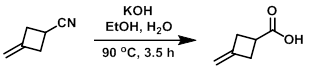
To a solution of KOH (10 g, 178 mmol) in H2O (15 mL) and EtOH (15 mL) at RT was added the SM (3.92 g, 42 mmol) over 10 min. The reaction mixture was stirred at 90 C for 3.5 h, after which time it was concentrated in vacuo. The resulting residue was dissolved in H2O (10 mL) and acidified at 0 C with 6M HCl to pH 1. The mixture was extracted with DCM. The org layer was dried (MgSO4) and concentrated to provide the product as a colorless oil which was taken forward without further purification. [4.65 g, 98%]
[Patent Reference: WO2015129926, page 108, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
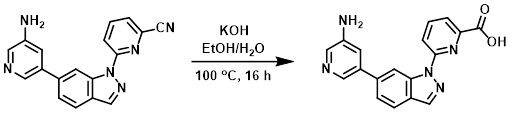
A mixture of the SM (60 mg, 0.19 mmol) and KOH (106 mg, 1.90 mmol) in 1:1 EtOH/H2O (2 mL) was stirred at 100 C for 16 h. After cooling to RT, the mixture was adjusted to pH = 7 using 3N HCl. The mixture was concentrated and purified by Prep HPLC [5-95%(ACN)/(0.5% TFA in H2O)] to provide the product as a white solid. [15 mg, 24%]
[Patent Reference: WO2016011390, page 78, ![]() (20.2 MB)]
(20.2 MB)]
Example 3
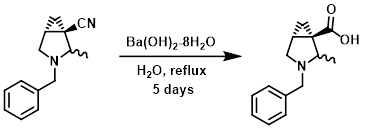
A mixture of the SM (2.4 g, 11 mmol) and Ba(OH)2-8H2O (5.3 g, 17 mmol) in H2O (100 mL) was heated at reflux for 5 days. The mixture was cooled to RT, then acidified via addition of 6N HCl. The mixture was concentrated in vacuo. The resulting residue was suspended in EtOH and filtered. The filtrate was concentrated and the residue was purified by silica gel flash chromatography to provide the product. [2.6 g, 99%]
[Patent Reference: WO2016014463, page 68, ![]() (6.7 MB)]
(6.7 MB)]
Example 4
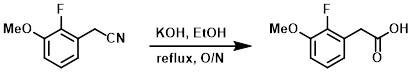
To a solution of the SM (15.4 g, 93 mmol) in EtOH (100 mL) was added KOH (12.3 g, 186 mmol). The reaction mixture was stirred at reflux overnight, after which time it was concentrated in vacuo and diluted with H2O. The mixture was acidified to pH = 1 with conc. HCl, causing a precipitate to form. The precipitated solids were filtered, recrystallized, and filtered again. The filter cake was washed with cold H2O and dried in a vac oven at 40 C overnight to provide the product. [11.5 g]
[Patent Reference: WO2016014463, page 126, ![]() (6.7 MB)]
(6.7 MB)]
