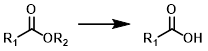
Ester to Acid
Common Conditions:
NaOH + H2O/MeOH
Other hydroxide sources (ex. KOH or LiOH) can be used in place of NaOH. For the hydrolysis of ethyl esters EtOH is often used in place of MeOH.
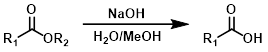
NaOH + H2O/THF
Other hydroxide sources (ex. KOH or LiOH) can be used in place of NaOH. Dioxane is sometimes used in place of THF. H2O/THF mixtures can be biphasic depending on the ratio of solvents, temperature, and ion concentration of the mixture.
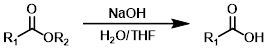
NaOH + H2O/MeOH/THF
Other hydroxide sources (ex. KOH or LiOH) can be used in place of NaOH. For the hydrolysis of ethyl esters EtOH is often used in place of MeOH. Dioxane is sometimes used in place of THF. H2O/MeOH/THF mixtures are monophasic.
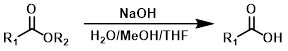
HCl (Acidic Conditions)
Ester hydrolysis under acidic conditions is a reversible process. A large excess of H2O is used to drive the equilibrium towards the carboxylic acid product (Fischer esterification is the opposite process).
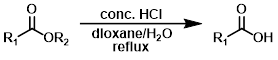
Reaction Map:
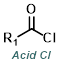
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
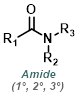 |
|||
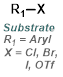 |
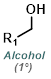 |
|||
 |
