Nitrile to Ester

Common Conditions:
Pinner Reaction
The Pinner reaction can be used to convert nitriles to esters. A wide range of nitriles can be substrates (ex. aliphatic, aromatic, and heteroaromatic nitriles). The most common alcohols employed are MeOH and EtOH, but many primary and secondary alcohols have been used.[1]
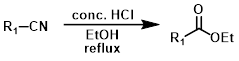
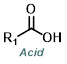
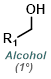
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
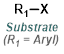 |
 |
 |
||
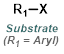 |
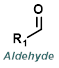 |
|||
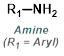 |
 |
References:
1) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis
