Nitrile to Ester
(Pinner Reaction)
Examples:
Example 1
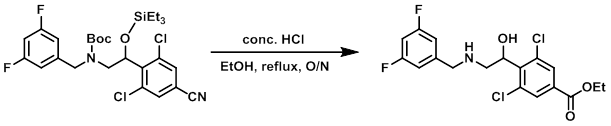
To a solution of the SM (0.2 g, 0.3 mmol) in EtOH (5 mL) was added conc. HCl (5 mL). The reaction mixture was stirred at reflux overnight. The mixture was quenched with H2O (50 mL), basified with 10% NaOH up to pH 9, and extracted with EtOAc (2 x 30 mL) The combined organics were washed with H2O (50 mL), brine (50 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (30% EtOAc/hexane) to provide the product as a white solid. [0.1 g, 92%]
[Patent Reference: WO2015129926, page 83, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
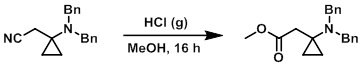
A solution of the SM (3.8 g, 13.8 mmol) in MeOH (40.0 mL) was saturated with dry HCl gas. The reaction was kept standing for 16 h, after which time the reaction mixture was concentrated in vacuo. The resulting material was stirred in H2O for 15 min, extracted with EtOAc, dried (Na2SO4), and concentrated in vacuo to provide the product as a yellow liquid. [4.0 g, 94%]
[Patent Reference: WO2014149164, page 234, ![]() (23.7 MB)]
(23.7 MB)]
Example 3
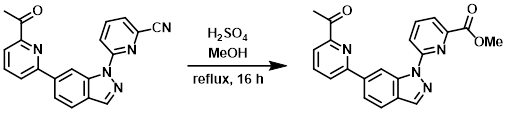
A solution of the SM (180 mg, 0.53 mmol) in MeOH (50 mL) and H2SO4 (2 mL) was refluxed for 16 h. After concentration, the residue was dissolved in DCM (100 mL) and adjusted to pH = 8 with 3N NaOH. The org phase was concentrated and the resulting residue was purified by silica gel chromatography (1:1 PE/EtOAc) to provide the product as a yellow solid. [118 mg, 60%]
[Patent Reference: WO2016011390, page 201, ![]() (20.2 MB)]
(20.2 MB)]
