Acid to Ester
(Fischer Esterification)
Examples:
Example 1
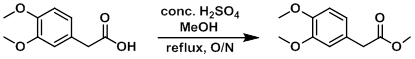
To a solution of the SM (25.0 g, 127.4 mmol) in MeOH (100 mL) was added a catalytic amount of H2SO4 (~10 drops). The mixture was refluxed overnight, after which time it was concentrated to remove MeOH and diluted with DCM (250 mL). The mixture was washed with H2O (5 x 20 mL), brine (20 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as an orange oil. [25.77 g, 96%]
[Patent Reference: WO2012112946, page 43 ![]() (11.2 MB)]
(11.2 MB)]
Example 2
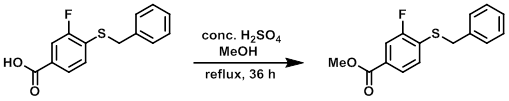
A suspension of the SM (81.4 g, 0.310 mol) in MeOH (800 mL) and conc. H2SO4 (2 mL) was heated to reflux for 36 h. The reaction mixture was cooled to RT and the resulting precipitate was filtered and washed with hexane. The filtrate was concentrated in vacuo and the resulting solid was filtered and washed with hexane. The combined solids were dried in vacuo to provide the product as a white solid. [60.9 g, 70%]
[Patent Reference: WO2010035166, page 73, ![]() (3.2 MB)]
(3.2 MB)]
Example 3
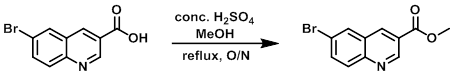
The SM (2.5 g, 9.92 mmol) was suspended in MeOH (40.2 mL, 991.8 mmol) and treated with conc. H2SO4 (2.64 mL, 49.59 mmol). The reaction was refluxed overnight, after which time it was cooled to RT and concentrated in vacuo. The residue was diluted with EtOAc and carefully neutralized with a solution of Na2CO3. The biphasic suspension was diluted with EtOAc/DCM until all the solid was dissolved. The org layer was separated, washed with brine, dried (Na2SO4), and concentrated under reduced pressure to provide the product.
[Patent Reference: WO2010038081, page 116, ![]() (33.8 MB)]
(33.8 MB)]
Example 4
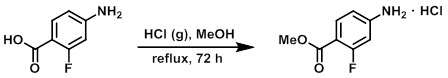
The SM (23.9 g, 0.154 mol) was dissolved in MeOH (500 mL) and HCl (g) was bubbled into the solution until the boiling point of the solution was reached. The reaction mixture was heated at reflux for 72 h. The solvent was evaporated to provide the product as a beige solid. [33.2 g, quantitative]
[Patent Reference: WO2010035166, page 69, ![]() (3.2 MB)]
(3.2 MB)]
Example 5
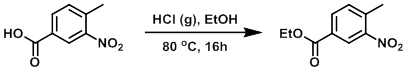
A solution of the SM (10 g, 55.2 mmol) in EtOH (120 mL) at -5 C was bubbled through with dry HCl gas for 10 min. The reaction mixture was stirred at 80 C for 16 h, after which time the mixture was concentrated in vacuo. The resulting material was dissolved in EtOAc (200 mL) and washed with cold sat aq NaHCO3 (2 x 200 mL), then H2O (200 mL). The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a colorless liquid. [10.5 g, 91%]
[Patent Reference: WO2014149164, page 299, ![]() (23.7 MB)]
(23.7 MB)]
Example 6
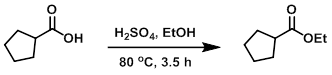
To a solution of the SM (1.14 g, 10 mmol) in EtOH (5 mL) was added H2SO4 (0.1 mL) at RT. The reaction mixture was stirred at 80 C for 3.5 h. The mixture was cooled to RT and poured into sat aq NaHCO3 (40 mL). The mixture was stirred at RT for 30 min, then extracted with EtOAc. The org layer was dried (MgSO4) and concentrated in vacuo to provide the product as a pale yellow oil which was used in the next step without further purification.
[Patent Reference: WO2015129926, page 105, ![]() (21.5 MB)]
(21.5 MB)]
Example 7
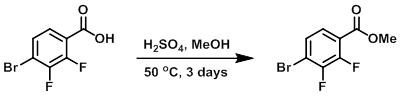
H2SO4 (1.1 mL, 21 mmol) was slowly added to a solution of the SM (2.5 g, 10.5 mmol) in MeOH (40 mL). The mixture was stirred at 50 C for 3 days. The mixture was concentrated in vacuo and the resulting material was partitioned between EtOAc and H2O. The mixture was basified with K2CO3. The org layer was washed with brine, dried (MgSO4), and concentrated to provide the product as a colorless oil which crystallized to a white solid. [2.6 g, 98%]
[Patent Reference: WO2015144799, page 202, ![]() (18.8 MB)]
(18.8 MB)]
