Ester to Acid
(NaOH + H2O/MeOH/THF)
Examples:
Example 1
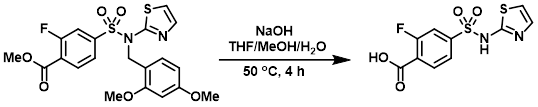
A mixture of the SM (10 g, 21 mmol) and NaOH(s) (4.3 g, 107 mmol) in THF/MeOH/H2O (25 mL, 2 mL, 75 mL) was stirred at 50 C for 4 h. The reaction mixture was acidified to pH 2 using aq 2N HCl and the resulting precipitate was filtered then purified by silica gel column chromatography (90:10:1 DCM/MeOH/NH3) to provide the product as a yellow solid. [706 mg]
[Patent Reference: WO2010035166, page 71, ![]() (3.2 MB)]
(3.2 MB)]
Example 2
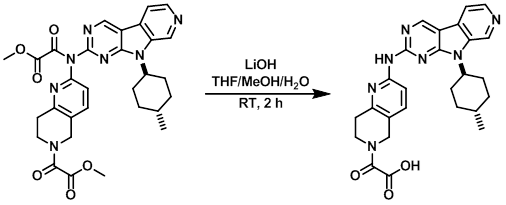
To a solution of crude SM in THF/MeOH/H2O (3:1:1, 5 mL) was added LiOH (19 mg, 0.80 mmol). The resulting mixture was stirred at RT for 2 h, after which time it was concentrated, the residue dissolved in acidic H2O, and purified by reverse phase Prep-HPLC. The resulting fractions were combined and lyophilized to provide the product as a light yellow solid. [32 mg, 33% over 2 steps]
[Patent Reference: WO2012129344, page 141, ![]() (7.3 MB)]
(7.3 MB)]
Example 3
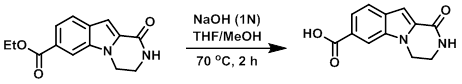
To a solution of the SM (270 mg, 1.04 mmol) in 1:1 THF/MeOH (10 mL) was added dropwise 1N NaOH (3 mL). The reaction mixture was stirred at 70 C for 2 h. After completion, the reaction mixture was cooled to 0 C, diluted with H2O (10 mL), and acidified to pH 2 with 3N HCl (10 mL). The resulting white precipitate was filtered, washed with H2O and MeOH, and dried in vacuo to provide the product as an off-white solid. [170 mg, 71%]
[Patent Reference: WO2014149164, page 301, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
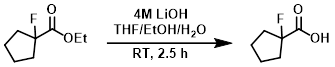
To a solution of the SM (910 mg, 5.68 mmol) in 2:4:1 THF/EtOH/H2O (7 mL) was added a solution of aq 4M LiOH (3 mL, 12 mmol). The reaction mixture was stirred at RT for 2.5 h. The org solvent was removed in vacuo. The resulting mixture was acidifed to pH 1 with 2M HCl, then extracted with EtOAc. The org layer was dried (MgSO4) and concentrated to provide the product as a brown oil which was taken forward without further purification. [709 mg, 95%]
[Patent Reference: WO2015129926, page 106, ![]() (21.5 MB)]
(21.5 MB)]
