Acid to Alcohol
1) Activation 2) NaBH4
Examples:
Example 1
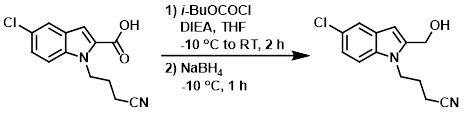
The SM (39.4 g, 149.98 mmol) and DIEA (51.69 mL, 300 mmol) were dissolved in THF (550 mL) and stirred at -10 C under N2. Then a solution of isobutylchloroformate in THF (50 mL) was added dropwise and stirring was continued for 1 h at -10 C, then 1 h at RT. NaBH4 (17.02 g, 450 mmol) was added portionwise at -10 C and stirred for 1 h. H2O (200 mL) was added cautiously to the reaction mixture and stirring was continued for another hour at RT under N2. The mixture was neutralized with 10% citric acid (aq) and then was extracted with EtOAc. The org layer was dried (MgSO4) and concentrated. The residue was purified by silica gel chromatography (50:50:0 to 0:100:0 to 0:99:1 heptane/DCM/MeOH) to provide the product as a white powder. [23.9 g, 64%]
[Patent Reference: WO2015158653, page 35, ![]() (2.9 MB)]
(2.9 MB)]
Example 2
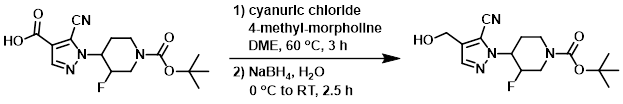
Freshly recrystallized (from heptane) cyanuric chloride (78 mg, 0.414 mmol) was dissolved in DME (2 mL), and 4-Methylmorpholine (0.020 mL, 0.215 mmol) was added. To this gummy solution was added the SM (70 mg, 0.21 mmol) dissolved in DME (2 mL). The reaction was stirred at 60 C for 3 h. The mixture was cooled to RT, filtered through a pad of celite, and the pad washed with DME. The filtrate was cooled to 0 C and a solution of NaBH4 (17 mg, 0.474 mmol) dissolved in H2O (0.4 mL) was added very slowly (dropwise). The resulting mixture was allowed to warm to RT for 2.5 h, after which time it was diluted with H2O and acidified to pH 2.5 using 1M NaHSO4. The aq layer was extracted with EtOAc (2x). The combined organics were dried (Na2SO4) and concentrated. The resulting material was purified by flash chromatography (eluting with 10-100% EtOAc/heptane) to provide the product as an oil. [28 mg, 42%]
[Patent Reference: WO2012069948, page 78, ![]() (3.9 MB)]
(3.9 MB)]
