Borane Dimethylsulfide
Other Names:
Borane-dimethyl sulfide complex
Dimethyl sulfide borane
(Dimethyl sulfide)trihydroboron
BMS
General Information:
Structure:
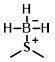
CAS Number: 13292-87-0
Molecular Weight: 75.96 g/mol
Appearance: Colorless liquid
Melting Point: -38 C
Density: 0.801 g/mL at 25 C
Borane dimethylsulfide (BMS) is a complex of borane with dimethylsulfide. The other common complex of borane is borane-THF. BMS is more stable than borane-THF and is therefore available in higher concentrations. BMS has the drawback of being smelly due to its dimethylsulfide content.
Common Uses:
Reagent for the reduction of carboxylic acids to alcohols
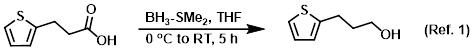
Procedure excerpt:
To a solution of the SM (1.0 g, 6.4 mmol) in THF (18 mL) at 0 C was added BH3-SMe2 (6.4 mL, 12.8 mmol). The reaction mixture was stirred at 0 C for 2 h, then RT for 3 h. . . .
Reagent for the reduction of esters to alcohols
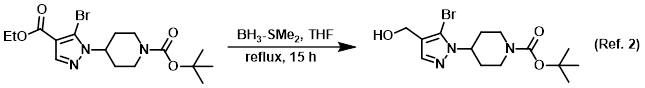
Procedure excerpt:
To a solution of the SM (137 g, 0.34 mol) in THF (1.3 L) at 0 C was slowly added BH3-SMe2 (97 mL, 1.02 mol). The reaction mixture was allowed to warm to RT then . . .
Reagent for the reduction of nitriles to amines
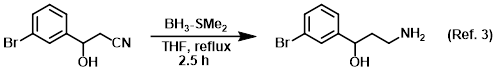
Procedure excerpt:
To a solution of the SM (117.5 g, 519.8 mmol) in dry THF under argon was slowly added BH3-SMe2 (68 mL, 675.7 mmol) over 30 min via dropping funnel. The reaction . . .
Safety:
Borane dimethylsulfide (BMS) is a flammable liquid.
References:
1) Patent Reference: WO2011061214, page 87, ![]() (8.2 MB)
(8.2 MB)
2) Patent Reference: WO2012069948, page 55, ![]() (3.9 MB)
(3.9 MB)
3) UK Pat App GB 1463151A, page 132
4) Wikipedia: Borane dimethylsulfide (link)
5) www.alfa.com: L07705 Borane-dimethyl sulfide complex, 94% (link)
6) www.tcichemicals.com: Dimethyl Sulfide Borane (link)
7) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
