Acid to Alcohol
(BH3-THF)
Examples:
Example 1
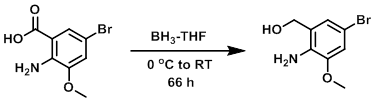
To a 0.24M THF suspension of the SM (71.7 mmol) at 0 C was added BH3-THF (1M, 220 mmol). The reaction mixture was stirred under Ar at RT for 66 h then quenched by addition of EtOH (15 mL) at 0 C and stirred an additional 15 min. The mixture was poured into H2O and extracted with DCM. The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the crude product as a white solid. [10.16 g, 62%]
[Patent Reference: WO2007117607, page 308, ![]() (12.9 MB)]
(12.9 MB)]
Example 2
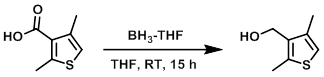
To a solution of the SM (246 mg, 1.5 mmol) in THF (3 mL) at 0 C was added dropwise BH3-THF (1M in THF, 5.5 mL, 5.5 mmol) over 15 min. The reaction mixture was stirred at RT for 15 h, after which time it was quenched with sat aq NaHCO3 and extracted with EtOAc (2 x 30 mL). The combined organics were washed with brine (2 x 10 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (25% EtOAc/hexane) to provide the product as a colorless gum. [201 mg, 90%]
[Patent Reference: WO2015129926, page 79, ![]() (21.5 MB)]
(21.5 MB)]
Example 3
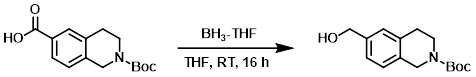
To a solution of the SM (12.50 g, 45.08 mmol) in dry THF (125 mL) under N2 at RT was added via syringe BH3-THF (99.17 mL, 99.17 mmol). The reaction mixture was stirred at RT for 16 h, after which time was slowly added H2O (10 mL) followed by 2M Na2CO3 (15 mL). The mixture was stirred for 15 min, then diluted with EtOAc. The layers were separated and the org layer was washed with 1M HCl, dried (MgSO4), and concentrated. The resulting oil was purified by silica gel chromatography to provide the product as a white solid. [11.8 g, 99.3%]
[Patent Reference: WO2016014463, page 88, ![]() (6.7 MB)]
(6.7 MB)]
