Alcohol to Bromide
(PBr3)
Examples:
Example 1

To a solution of the SM (1.12 g, 6.66 mmol) in dry ether (19 mL) at 0 C was added dropwise PBr3 (0.626 mL, 6.66 mmol). The ice bath was removed and the reaction was stirred for 3 h. H2O was carefully added to the mixture and the layers were separated. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo to provide the product. [1.49 g, 97%]
[Patent Reference: WO2015144799, page 189, ![]() (18.8 MB)]
(18.8 MB)]
Example 2
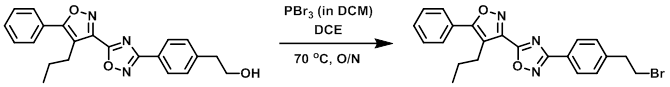
To a mixture of the SM (400 mg, 1.065 mmol) in DCE (20 mL) was added PBr3 in DCM (1.065 mL, 1.065 mmol). The reaction mixture was stirred at 70 C overnight, after which time it was diluted with DCM. The mixture was washed with 1N NaOH, dried (MgSO4), concentrated, and purified by silica column (0-50% EtOAc/hexane, 40 g column) to provide the product. [77 mg]
[Patent Reference: WO2011017578, page 185, ![]() (8.3 MB)]
(8.3 MB)]
Example 3
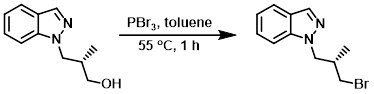
To a stirring solution of the SM (0.666 g, 3.50 mmol) in toluene (7.0 mL) was added PBr3 (0.329 mL, 3.50 mmol). The reaction mixture was stirred at 55 C for 1 h. The mixture was cooled to RT, quenched with sat aq NaHCO3, and extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine, dried (MgSO4), and concentrated. The resulting oil was purified by column chromatography (20% EtOAc/hexane) to provide the product as an oil. [0.200 g]
[Patent Reference: WO2014152144, page 56, ![]() (4.6 MB)]
(4.6 MB)]
