Bromine
General Information:
Structure:
![]()
CAS Number: 7726-95-6
Molecular Weight: 159.81 g/mol
Appearance: Brown/red fuming liquid
Chemical Formula: Br2
Melting Point: -7.2 C
Boiling Point: 58.8 C
Density: 3.119 g/mL
Bromine (Br2) is typically used in bromination reactions. As a fuming brown/red liquid, bromine can be difficult to handle on small or large scale. A common, easy to handle alternative to bromine is N-Bromosuccinimide (NBS).
Common Uses:
Reagent in SEAr (Electrophilic Aromatic Substitution) brominations
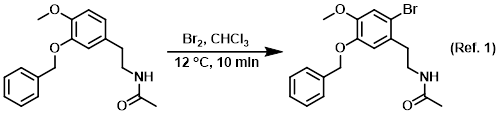
Procedure excerpt:
To a solution of the SM (80 g, 0.27 mol) in CHCl3 (1 L) at 10-15 C was added dropwise a solution of Br2 (16 mL, 0.31 mol) in CHCl3 (100 mL). The reaction was stirred . . .
Reagent in brominations at alpha positions
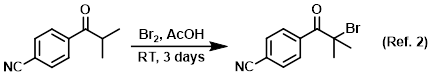
Procedure excerpt:
To a mixture of the SM (2.4 g, 13.86 mmol) in AcOH (50 mL) was added Br2 (0.80 mL, 15.53 mmol). The mixture was stirred at RT for 3 days, after which time . . .
Reagent in the conversion of alcohols to bromides via Appel reactions
![]()
Procedure excerpt:
To a solution of PPh3 (26.2 g, 0.10 mol) in ACN (50 mL) at 0 C was added dropwise a solution of Br2 (5.13 mL, 0.10 mol) in ACN (30 mL). The SM (5.1 g, 0.050 mol) was . . .
Safety:
Bromine is a very toxic and corrosive volatile liquid. Skin contact can cause painful burns and blisters.
References:
1) Patent Referenc: WO2014177977, page 64, ![]() (6.0 MB)
(6.0 MB)
2) Patent Reference: WO2011017578, page 154, ![]() (8.3 MB)
(8.3 MB)
3) Patent Reference: WO2015129926, page 100, ![]() (21.5 MB)
(21.5 MB)
4) Wikipedia: Bromine (link)
5) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
