Stille
Examples:
Example 1
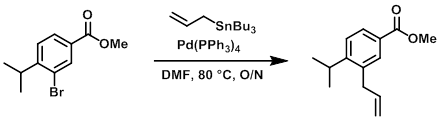
To a solution of the bromide (1.2 g, 4.7 mmol) in dry DMF (36 mL) degassed with N2 was added Pd(PPh3)4 (270 mg, 0.23 mmol) and the stannane (1.85 g, 5.6 mmol). The mixture was flushed with N2 for 5 min, then stirred at 80 C overnight. After cooling, the mixture was partitioned between EtOAc and brine. The org layer was washed with brine (2x), dried, and concentrated to give 3.5 g of a yellow oil. The material was purified by Prep LC (80 g silica, 5-10% EtOAc/heptane) to provide the product as a colorless oil. [900 mg, 88%]
[Patent Reference: WO2015144799, page 105, ![]() (18.8 MB)]
(18.8 MB)]
Example 2
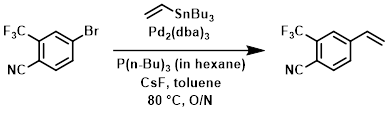
To a mixture of the bromide (500 mg, 2.00 mmol), CsF (668 mg, 4.40 mmol), P(n-Bu)3 in hexane (0.347 mL, 0.120 mmol), Pd2(dba)3 (36.6 mg, 0.040 mmol), and toluene (10 mL) was added the stannane (0.587 mL, 2.00 mmol). The reaction mixture was stirred at 80 C overnight, after which time was added a saturated KF solution. The resulting mixture was stirred 1 h, filtered, and the filtrate diluted with EtOAc. The org mixture was washed with H2O, dried (MgSO4), and concentrated. The resulting solids were purified by silica gel column chromatography (EtOAc/hexane) to provide the product. [450 mg]
[Patent Reference: WO2011017578, page 129, ![]() (8.4 MB)]
(8.4 MB)]
Example 3
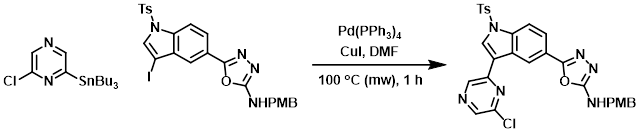
To a 20 mL glass microwave tube was added the stannane (807 mg, 1.999 mmol), the iodo compound (1.00 g, 1.665 mmol), CuI (381 mg, 1.999 mmol), and Pd(PPh3)4 (96 mg, 0.083 mmol). The mixture was purged with argon, then DMF (15 mL) was added. The tube was heated in a microwave reactor at 100 C for 1 h. The mixture was diluted with DCM and washed with H2O. The org layer was dried and concentrated. The resulting material was purified by flash chromatography to provide the product as a light yellow amorphous solid. [0.48 g, 49%]
[Patent Reference: WO2012129338, page 75, ![]() (12.0 MB)]
(12.0 MB)]
Example 4
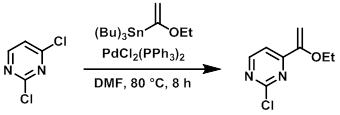
To a solution of the chloride (3.0 g, 20.0 mmol) in dry DMF (30 mL) was added the stannane (14.0 g, 40.0 mmol), and the mixture was degassed for 10 min. To this mixture was added PdCl2(PPh3)2 (0.7 g, 1.0 mmol), and the mixture was again degassed for 5 min. The reaction mixture was stirred at 80 C for 8 h, after which time TLC indicated complete consumption of the SM. The mixture was quenched with H2O, extracted with EtOAc, dried (Na2SO4), and concentrated in vacuo to provide the product which was taken forward without further purification.
[Patent Reference: WO2014149164, page 339, ![]() (23.7 MB)]
(23.7 MB)]
Example 5
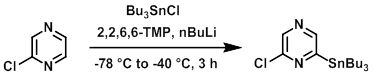
To a solution of nBuLi (1.6 M in hexane, 21.49 mL, 34.4 mmol) in THF (80 mL) at -50 C was added 2,2,6,6-tetramethylpiperidine (5.80 mL, 34.4 mmol). The mixture was warmed to 0 C and stirred 20 min. The mixture was then cooled to -78 C. In another flask, the SM (0.974 mL, 10.91 mmol) and Bu3SnCl (2.96 mL, 10.91 mmol) were dissolved in THF (50 mL) and cooled to -78 C. This cooled solution was transferred to the lithium tetramethylpiperidide solution via cannula, and the resulting orange mixture was slowly warmed to -40 C over 3 h. The mixture was quenched with saturated HCl:EtOH:THF (1:4:5, 50 mL) and warmed to RT. The mixture was neutralized with saturated NaHCO3 and the volatiles were removed in vacuo. The resulting material was diluted with H2O and extracted with DCM. The combined organics were dried and concentrated. The resulting residue was purified by silica gel chromatography (eluting with 0-30% DCM/hexanes) to provide the product as a clear oil. [3.15 g, 71.5%]
[Patent Reference: WO2012129338, page 74, ![]() (12.0 MB)]
(12.0 MB)]
