Cesium Fluoride
Other Names:
Caesium fluoride
General Information:
Structure:
![]()
CAS Number: 13400-13-0
Molecular Weight: 151.90 g/mol
Appearance: White solid
Chemical Formula: CsF
Melting Point: 682 C
Boiling Point: 1251 C
Cesium fluoride (CsF) has higher solubility than NaF or KF. Cesium is less electronegative than sodium or potassium and is larger in size, both properties which allow for easier dissociation from the fluorine ion. Cesium fluoride is less hygroscopic than other sources of highly dissociated fluorine such as TBAF. The moderately basic nature of fluorine also makes it useful as a base in certain reactions, especially when a non-nucleophilic base is desired.
Common Uses:
Base for palladium catalyzed reactions (ex. Stille reactions)
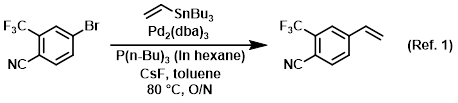
Procedure excerpt:
To a mixture of the bromide (500 mg, 2.00 mmol), CsF (668 mg, 4.40 mmol), P(n-Bu)3 in hexane (0.347 mL, 0.120 mmol), Pd2(dba)3 (36.6 mg, 0.040 mmol), and toluene . . .
Safety:
Cesium fluoride (CsF) is a hygroscopic solid. It is harmful if inhaled or swallowed. Contact with acid can form hydrofluoric acid.
References:
1) Patent Reference: WO2011017578, page 129, ![]() (8.3 MB)
(8.3 MB)
2) Wikipedia: Caesium fluoride (link)
3) www.sigmaaldrich.com: Cesium fluoride (link)
4) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
