Amine to Amide
(HATU)
Examples:
Example 1
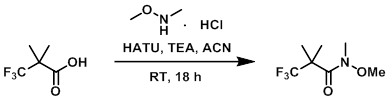
To a solution of the acid (5.00 g, 32.0 mmol) in ACN (22.9 mL) was added TEA (9.82 mL, 70.5 mmol) followed by HATU (12.8 g, 33.6 mmol). The mixture was stirred at RT for 15 min, after which time the dark clear mixture was treated with the amine (3.44 g, 35.2 mmol). The reaction mixture was stirred at RT for 18 h. The mixture was diluted with EtOAc (100 mL) and washed with 1N HCl (2 x 100 mL), sat. NaCl (5 x 100 mL), dried (Na2SO4), and concentrated. The resulting crude orange solid was adsorbed onto a plug of silica gel and purified by chromatography (0-25% EtOAc/heptane) to provide the product as a yellow liquid. [5.050 g, 79%]
[Patent Reference: WO2015129926, page 124, ![]() (21.5 MB)]
(21.5 MB)]
Example 2

To a solution of the amine (0.18 g, 0.5 mmol), HATU (0.4 g, 1.1 mmol), and DIEA (0.5 mL, 4.0 mmol) in DMA (5 mL) was added the acid (0.1 mL, 1.05 mmol). The reaction was stirred at 60 C for 2h, after which time it was diluted with EtOAc and washed with 10% LiCl (3x) and brine (1x). The org layer was dried (Na2SO4), concentrated, and purified by silica gel column chromatography (5% MeOH/DCM) to provide the product as a white solid. [0.1 g, 49%]
[Patent Reference: WO2007089768, page 233, ![]() (20.6 MB)]
(20.6 MB)]
Example 3
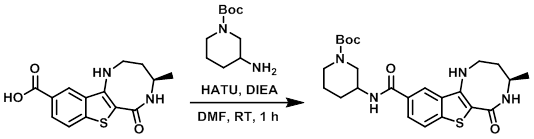
To a solution of the acid (200 mg, 0.7 mmol) in DMF (3.0 mL) at 0 C was added HATU (550 mg, 1.4 mmol) and DIEA (280 mg, 2.1 mmol), followed by the addition of the amine (173 mg, 0.8 mmol). The resulting mixture was stirred at RT for 1 h, after which time the reaction mixture was diluted with H2O and extracted with EtOAc. The org layer was dried (Na2SO4) and concentrated in vacuo. The resulting material was triturated in n-pentane to provide the product as a brown gummy solid. [250 mg, 63%]
[Patent Reference: WO2014149164, page 278, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
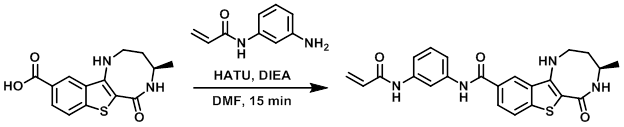
To a solution of the acid (120 mg), amine (1.1 equiv), DIEA (3 equiv), and DMF (1 mL) was added HATU (1.1 equiv). The reaction mixture was stirred for 15 min, after which time it was diluted with H2O (0.5 mL) and purified directly by prep-HPLC to provide the TFA salt of the product as an off-white solid. [48.0 mg, 39%]
[Patent Reference: WO2014149164, page 261, ![]() (23.7 MB)]
(23.7 MB)]
Example 5
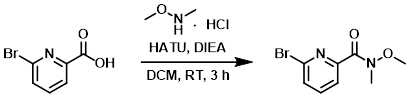
To a solution of the acid (68 g, 0.34 mol) in DCM (1 L) was added the amine (39 g, 0.40 mol), HATU (152 g, 0.40 mol), and DIEA (130.3 g, 1.01 mol). The reaction was stirred at RT for 3 h. The mixture was washed with 1N HCl (2 x 600 mL), dried (Na2SO4), and concentrated. The crude residue was purified by silica gel chromatography (10-30% EtOAc/hexane) to provide the product as a light colored oil. [80 g, 97%]
[Patent Reference: WO2015140133, page 83, ![]() (11.7 MB)]
(11.7 MB)]
Example 6
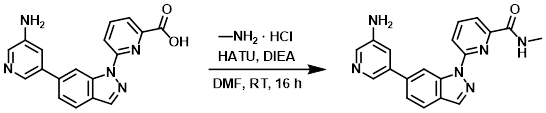
A mixture of the SM (38 mg, 0.11 mmol), DIEA (43 mg, 0.33 mmol), and HATU (65 mg, 0.17 mmol) in DMF (2 mL) was stirred at RT for 20 min. Methylamine-HCl (11 mg, 0.17 mmol) was added and the reaction mixture was stirred at RT for 16 h. The mixture was purified by Prep HPLC [5-95% (ACN)/(0.5% NH4OH in H2O)] to provide the product as a white solid. [11 mg, 28%]
[Patent Reference: WO2016011390, page 79, ![]() (20.2 MB)]
(20.2 MB)]
Example 7
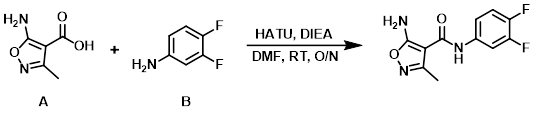
A mixture of the acid (A) (3.00 g, 21.1 mmol), the amine (B) (2.09 mL, 21.1 mmol), DIEA (10.5 mL, 63.3 mmol), and HATU (8.03 g, 21.1 mmol) in DMF (130 mL) was stirred at RT overnight. The reaction mixture was partitioned between DCM and H2O. Phase separation was achieved via Whatman filter. The org layer was concentrated in vacuo and the resulting crude material was purified by Prep MPLC (Biotage Isolera, 100 g SNAP cartridge, 10-80% EtOAc/hexane) to provide the product. [2.40 g, 45%]
[Patent Reference: WO2016012477, page 162, ![]() (8.1 MB)]
(8.1 MB)]
