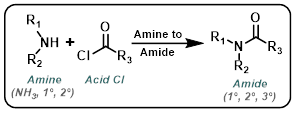
Amine to Amide
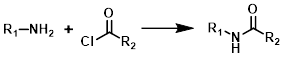
Common Conditions:
Acid Cl + Amine
The reaction of acid chlorides and amines to form amides is very general. Typically the reaction takes place at RT with a suitable base (ex. TEA or DIEA) in an aprotic solvent (ex. DCM, THF, or DMF).

1) SOCl2 2) Amine
Starting from the carboxylic acid a two step process is often used. Thionyl chloride (SOCl2) or oxalyl choride [(COCl)2] is used to make the acid chloride. The crude acid chloride is then isolated and reacted with the amine in a similar manner as described above.

Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
| For Amine: | ||||
 |
||||
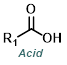 |
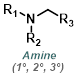 |
|||
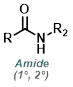 |
||||
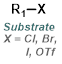 |
||||
| For Acid Cl: | ||||
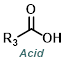 |
||||
