Amine to Amide
1) SOCl2 2) Amine
Examples:
Example 1
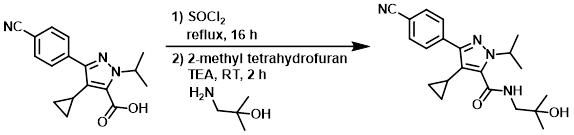
SOCl2 (20 mL) was added to the SM (2.50 g, 8.47 mmol) and the mixture was refluxed for 16 h. The volatiles were removed in vacuo and the mixture was azeotroped with toluene (3 x 50 mL) to give a gum. To the crude mixture was added 2-methyl tetrahydrofuran (20 mL), followed by TEA (2.36 mL, 17 mmol) and the amine (907 mg, 10.2 mmol). The mixture was stirred at RT for 2 h, quenched with sat aq NaHCO3 (20 mL), and extracted with EtOAc (2 x 20 mL). The combined organics were washed with 10% aq citric acid (20 mL) and concentrated in vacuo to give a gum. The crude was triturated in ether to provide to product as a white solid. [2.32 g, 75%]
[Patent Reference: WO2010032200, page 70, ![]() (6.2 MB)]
(6.2 MB)]
Example 2
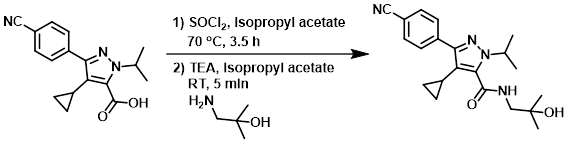
SOCl2 (16.6 mL, 203 mmol) was added to the SM (60 g, 203 mmol) in isopropyl acetate (600 mL). The reaction mixture was stirred at 70 C for 3.5 h, then allowed to cool to RT. To the mixture was slowly added TEA (62.3 mL, 447 mmol) followed by the slow addition of the amine (21.7 g, 244 mmol) in isopropyl acetate (200 mL) over 20 min. The reaction was stirred at RT for 5 min then diluted with saturated aq NaHCO3 (600 mL) and H2O (500 mL). The resulting mixture was extracted with EtOAc (1.7 L) and the org layer washed with aq KOH (1 N, 3 x 200 mL). The org layer was dried (MgSO4) and concentrated to give a brown solid. The crude was recrystallized from ACN/H2O (9:1, 660 mL) and the resulting solid was filtered, washed with H2O (70 mL), and dried to provide to product as a white solid. [42 g, 56%]
[Patent Reference: WO2010032200, page 71, ![]() (6.2 MB)]
(6.2 MB)]
Example 3
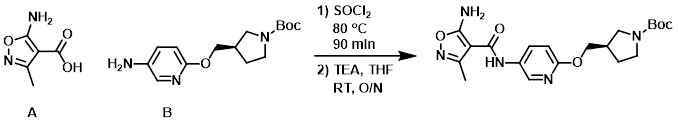
A mixture of the acid (A) (0.9 g, 6.3 mmol) in SOCl2 (5 mL, 69 mmol) was stirred at 80 C for 90 min. After cooling, the volatiles were removed in vacuo. The crude acid chloride was diluted with toluene and concentrated (this process was repeated two more times). The acid chloride was then dissolved in THF (16 mL), TEA (2.4 mL, 17.2 mmol) was added, followed by the dropwise addition of a solution of the amine (B) (1.7 g, 5.7 mmol) in THF (16 mL). The reaction mixture was stirred at RT overnight. The mixture was concentrated in vacuo and the resulting crude material was purified by Prep MPLC (Biotage Isolera, 55 g NH-cartridge, 0-6% EtOH/DCM) to provide the product. [1.8 g, 75%]
[Patent Reference: WO2016012477, page 156, ![]() (8.1 MB)]
(8.1 MB)]
Example 4
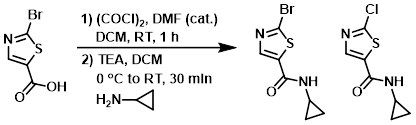
A suspension of the acid (1.07 g, 5.14 mmol) in DCM (25 mL) at 0 C was treated with (COCl)2 (0.64 mL, 7.20 mmol) followed by DMF (3 drops). The mixture was stirred at RT for 1 h, after which time it was concentrated in vacuo. The resulting solids were again suspended in DCM (25 mL), cooled in an ice/H2O bath, and treated with TEA (0.72 mL, 5.14 mmol) followed by the amine (0.54 mL, 7.72 mmol). The reaction was stirred at 0 C for 15 min, then RT for 15 min. The mixture was then diluted with DCM (100 mL), washed with 1N NaOH (30 mL), then brine (10 mL). The org layer was dried and concentrated to provide the product as a 1:1 mixture of the bromide and chloride which was used in the next step without further purification.
[Patent Reference: WO2012129338, page 230, ![]() (12.0 MB)]
(12.0 MB)]
