Amide to Nitrile

Common Conditions:
POCl3
Phosphorus oxychloride (POCl3), with heating, readily converts unsubstituted amides to nitriles. HCl forms during the reaction. Acid sensitive substrates may not be well-tolerated.[1]
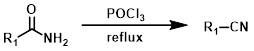
SOCl2
Thionyl chloride (SOCl2), with heating, readily converts unsubstituted amides to nitriles. Sulfur dioxide (SO2) and HCl form during the reaction. Acid sensitive substrates may not be well-tolerated.[1]

TFAA
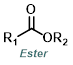
Trifluoroacetic anhydride (TFAA) provides milder, non-acidic conditions. One drawback is the possible trifluoroacetylation of reactive functionalities (ex. amines).
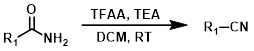
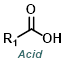
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
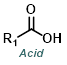 |
||||
 |
||||
 |
 |
 |
||
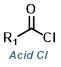 |
 |
|||
 |
||||
 |
References:
1) Caron, S.; Practical Synthetic Organic Chemistry
