Mitsunobu
(Azide)
Examples:
Example 1
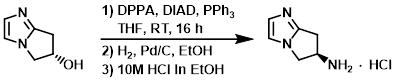
To a solution of the SM (48 g, 384 mmol) and PPh3 (121 g, 461 mmol) in THF (1.8 L) was added DIAD ( 93.1 g, 461 mmol). The reaction mixture was cooled to 10 C and treated with DPPA (98.8 g, 461 mmol). The mixture was stirred at RT for 16 h and then concentrated in vacuo. The crude was purified by flash chromatography (eluting with 33% EtOAc/pentane). The product fractions were concentrated to ~150 mL and diluted with EtOH (1.0 L). 10% Pd/C was added and the mixture was hydrogenated under 1 atm of H2. The mixture was filtered and the filtrate was concentrated in vacuo to a volume of ~500 mL.10M HCl in EtOH (50 mL) was added dropwise and the resulting solid was filtered and washed with EtOH to provide the product as a solid. [30 g, 44%]
[Patent Reference: WO2010032200, page 158, ![]() (6.2 MB)]
(6.2 MB)]
Example 2
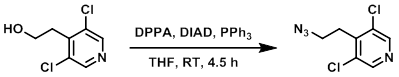
To a solution of the SM (3.1 g, 16.2 mmol) in THF (60 mL) at 0 C was added DIAD (6.3 mL, 32.0 mmol), PPh3 (8.52 g, 32.5 mmol), and DPPA (6.98 mL, 32.5 mmol). The reaction mixture was stirred at RT for 4.5 h. The mixture was quenched with H2O and extracted with EtOAc. The org layer was washed with brine (2x), dried (MgSO4), concentrated, and purified by silica gel column chromatography to provide the product as a yellow oil. [2.4 g, 68%]
[Patent Reference: WO2015129926, page 146, ![]() (21.5 MB)]
(21.5 MB)]
Example 3
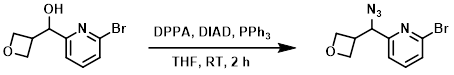
To a solution of PPh3 (317 mg, 1.21 mmol) in anhydrous THF (5 mL) at 0 C was added DIAD (224 mg, 1.11 mmol), followed by the SM (260 mg, 1.1 mmol) in anhydrous THF (2.6 mL), then DPPA (554 mg, 2.01 mmol). The reaction mixture was allowed to warm to RT and stir for 2 h. The mixture was diluted with ether and washed with sat aq NaHCO3, brine, dried (MgSO4), and concentrated. The resulting material was purified using a 12 g silica cartridge (15-25% acetone/heptane) to provide the product. [0.24 g, 84%]
[Patent Reference: WO2016011390, page 412, ![]() (20.2 MB)]
(20.2 MB)]
