Acetyl Protection
![]()
Common Conditions (Protection):
Acetyl Chloride
Acetyl protection of amines can be easily carried out with acetyl chloride (AcCl) in the presence of a base (ex. TEA or DIEA) with an appropriate solvent (ex. THF or DCM).[1]

Common Conditions (Deprotection):
Basic Conditions
Acetamides can be deprotected in the presence of a hydroxide base (ex. KOH or NaOH) in an appropriate solvent (ex. EtOH/H2O) at elevated temperatures (ex. reflux) . These harsh conditions may not be compatible with base sensitive substrates.

Acidic Conditions
Acetamides can be deprotected in the presence of a strong acid (ex. HCl) in an appropriate solvent (ex. EtOH/H2O) at elevated temperatures (ex. reflux). These harsh conditions may not be compatible with acid sensitive substrates.[1]
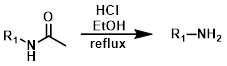
References:
1) Wuts, P. G. M.; Greene, T. W.; Greene's Protective Groups in Organic Synthesis, 4th Edition
