Mitsunobu
(N-Heteroaryls)
Examples:
Example 1
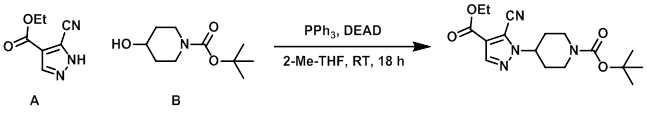
A (50 g, 300 mmol), B (67 g, 333 mmol), and PPh3 (111 g, 420 mmol) were dissolved in 2-Me-THF (200 mL). The mixture was cooled to 0 C and treated dropwise with DEAD (40% in toluene, 76.5 mL, 420 mmol). The mixture was allowed to warm to RT over 1h, then was stirred at RT 18 h. Under vigorous stirring, heptane (1.4 L) was carefully added and a suspension formed after 1 h. The solids were filtered off, and the filter cake was washed with 2:1 heptane/EtOAc (600 mL). The filtrate was concentrated and the resulting material was purified by flash chromatography (eluting with 25% EtOAc/heptane) and then re-crystallized from EtOAc/heptane to provide the product. [35.2 g, 33%]
[Patent Reference: WO2012069948, page 115, ![]() (3.9 MB)]
(3.9 MB)]
Example 2
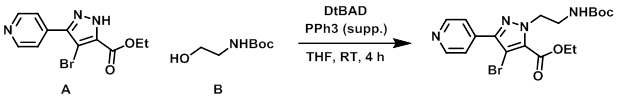
To a mixture of A (25 g, 84.4 mmol), B (20.4 g, 126 mmol), and PPh3 supp. (39.6 g, 127 mmol) in dry THF (700 mL) was added DtBAD (29.2 g, 126.6 mmol). The reaction mixture was stirred at RT for 4 h, after which time it was filtered through a glass frit. The filtrate was concentrated in vacuo to give 79.8 g of crude material that was purified by silica gel chromatography (330 g silica, 100% DCM to 97% DCM, 3% MeOH, 0.1% NH4OH) to provide the product. [33.2 g, 90%]
[Patent Reference: WO2015144799, page 84, ![]() (18.8 MB)]
(18.8 MB)]
Example 3
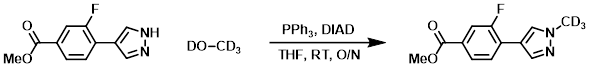
To a solution of the SM (160 mg, 0.73 mmol) in THF (5 mL) was added CDOD (26.2 mg, 0.73 mmol), PPh3 (229 mg, 0.872 mmol), and DIAD (0.18 mL, 0.95 mmol) at RT. The reaction mixture was stirred under N2 at RT overnight. The solvent was removed in vacuo and the residue was purified by normal phase chromatography to provide the product as a white solid. [92 mg, 53%]
[Patent Reference: WO2016010950, page 251, ![]() (18.8 MB)]
(18.8 MB)]
