Alkylation
Examples:
Example 1
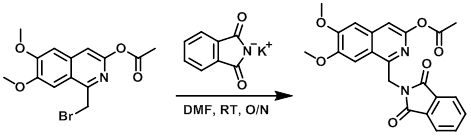
To a stirred solution of the SM (2.57 g, 7.56 mmol) in dry DMF (60 mL) was added potassium phthalimide (2.43 g, 13.12 mmol). The reaction mixture was stirred O/N at RT, after which time it was diluted with Et2O (150 mL) and H2O (40 mL). The resulting solid was filtered. The aq phase was isolated, diluted with Et2O (100 mL), stirred vigorously, and the resulting solids filtered (repeated 2x). All the solids were combined, washed with Et2O (100 mL), and dried under vacuum in the presence of P2O5 to provide the product was an off-white solid. [2.10 g, 68%] [WO2012112946, page 166]
Example 2
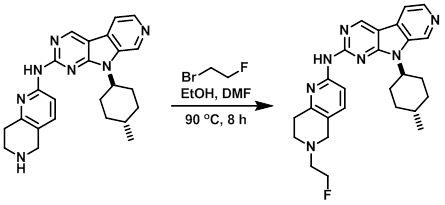
To a solution of the SM (149 mg, 0.36 mmol) in absolute EtOH (4 mL) and DMF (2 mL) was added 1-bromo-2-fluoroethane (50 uL, 0.67 mmole). The resulting mixture was stirred in a pre-heated oil bath at 90 C. After 70 min, a second aliquot of 1-bromo-2-fluoroethane (50 uL, 0.67 mmol) was added. After 7.5 h of heating LCMS indicated a majority consumption of the SM. The mixture was allowed to cool to RT and the volatiles were removed in vacuo. The resulting solution was diluted with DCM and washed sequentially with saturated aq Na2CO3, H2O, and brine. The org layers were dried over Na2SO4, concentrated, and purified by silica gel chromatography (10-45% 90:9:1 DCM/MeOH/NH4OH in DCM). The product fractions were combined, concentrated, and the resulting residue precipitated from DCM/hexanes. The suspension was sonicated, filtered, and the resulting solids dried under high vac overnight to provide the product as an off-white solid. [35 mg, 21%] [WO2012129344, page 145]
Example 3
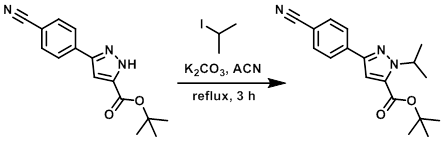
To a solution of the SM (15.7 g, 58.3 mmol) in ACN (200 mL) was added K2CO3 (16.1 g, 117 mmol) and 2-iodo-propane (8.73 mL, 87.4 mmol). The resulting mixture was stirred at reflux for 3 h. The reaction mixture was then partitioned between EtOAc (300 mL) and H2O (300 mL). The aqueous layer was further extracted with EtOAc (2 x 200 mL) and the combined organics washed with brine (200 mL), dried (MgSO4), and concentrated in vacuo to provide an off-white solid. The crude was purified by flash chromatography (eluting with 20% EtOAc/pentane) to provide the product as a white solid. [16.3 g, 90%] [WO2010032200]
Example 4
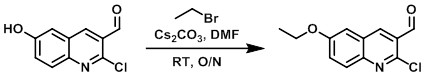
To a solution of the SM (0.70 g, 3.37 mmol) in dry DMF (15 mL) was added Cs2CO3 (1.21 g, 3.71 mmol). The resulting mixture was stirred at RT 10 min, after which time bromoethane (0.30 mL, 4.05 mmol) was added and the reaction mixture was stirred at RT O/N. The mixture was then diluted with Et2O (100 mL) and H2O (10 mL). The layers were separated and the org layer was further washed with H2O (2 x 10 mL), brine (20 mL), dried (Na2SO4), and concentrated in vacuo. The resulting crude was purified by silica gel chromatography (0-25% EtOAc/cyclohexane) to provide the product as a yellow solid. [544 mg, 68%] [WO2012112946, page 129]
