TBS Protection
(TBS-Cl)
Examples:
Example 1
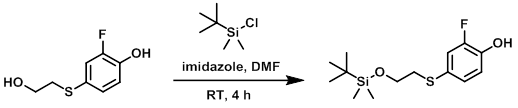
To a solution of the SM (985 mg, 5.24 mmol), imidazole (371 mg, 5.30 mmol), and DMF (5 mL) was added portionwise TBSCl (814 mg, 5.24 mmol). The reaction mixture was stirred at RT for 4 h. The mixture was concentrated in vacuo, diluted with H2O, and extracted with EtOAc (3x). The combined organics were washed with brine, dried (MgSO4), and concentrated to provide the product as an orange oil which was used without further purification. [1.43 g, 90%]
[Patent Reference: WO2012069948, page 52, ![]() (3.9 MB)]
(3.9 MB)]
Example 2
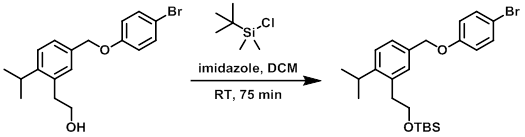
To a solution of the SM (1.2 g, 3.4 mmol), imidazole (0.70 g, 10 mmol), and dry DCM (33 mL) was added TBSCl (0.77 g, 5.1 mmol) under N2 at RT. The reaction mixture was stirred at RT for 75 min. The mixture was quenched with H2O and extracted with DCM. The layers were separated and the org layer was washed with H2O, brine, dried (MgSO4), and concentrated to provide the product as a colorless oil which was carried to the next step without further purification. [1.69 g, 100%]
[Patent Reference: WO2015144799, page 107, ![]() (18.8 MB)]
(18.8 MB)]
Example 3
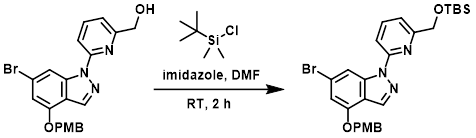
To a mixture of the SM (2.35 g, 5.36 mmol) and imidazole (729 mg, 10.72 mmol) in DMF (30 mL) at RT was added TBSCl (1.21 g, 8.04 mmol). The reaction mixture was stirred at RT for 2 h. The mixture was diluted with H2O (300 mL) and extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine (100 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography (5:1 PE/EtOAc) to provide the product as a yellow solid. [2.34 g, 79%]
[Patent Reference: WO2016011390, page 271, ![]() (20.2 MB)]
(20.2 MB)]
Example 4
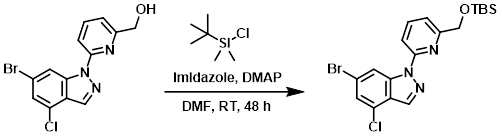
A mixture of the SM (0.750 g, 2.22 mmol), imidazole (0.302 g, 4.43 mmol), TBSCl (0.401 g, 2.66 mmol), and DMAP (0.01 g, 0.08 mmol) in DMF (6.86 mL) was stirred at RT for 48 h. The mixture was diluted with DCM, washed with NaHCO3, and concentrated. The residue was purified by silica gel chromatography (40 g silica, EtOAc/heptane) to provide the product as a clear solid. [600 mg]
[Patent Reference: WO2016011390, page 459, ![]() (20.2 MB)]
(20.2 MB)]
Example 5

A mixture of the SM (4.88 g, 31.2 mmol), TBSCl (7.06 g, 46.8 mmol), imidazole (4.25 g, 62.44 mmol), and THF (130 mL) was stirred at RT for 16 h. The mixture was concentrated in vacuo and the resulting residue was diluted with H2O (50 mL). The mixture was extracted with MTBE (50 mL). The org layer was washed with 1N HCl (50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo. The residue was purified by flash chromatography to provide the product. [8.23 g]
[Patent Reference: WO2016014463, page 141, ![]() (6.7 MB)]
(6.7 MB)]
Example 6

To a solution of the SM (1.49 g, 6.08 mmol) in DMF (10 mL) was added imidazole (0.621 g, 9.12 mmol) and TBS-Cl (1.10 g, 7.30 mmol) at 0 C. The reaction mixture was stirred under Ar at RT overnight. The mixture was diluted with EtOAc and washed with H2O and brine. The org layer was dried (Na2SO4) and concentrated. The crude material was purified by normal phase chromatography to provide the product. [1.89 g, 87%]
[Patent Reference: WO2016010950, page 246, ![]() (18.8 MB)]
(18.8 MB)]
Example 7
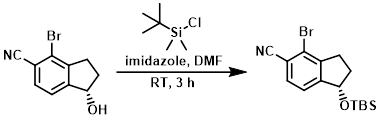
The SM (0.90 g, 3.78 mmol), imidazole (0.64 g, 9.45 mmol), and TBS-Cl (0.80 g, 5.29 mmol) were suspended in dry DMF and stirred at RT for 3 h. The mixture was diluted with H2O and extracted with EtOAc. The combined organics were dried and concentrated to provide the crude product which was used directly in the next step. [1.20 g]
[Patent Reference: WO2015078802, page 75, ![]() (3.8 MB)]
(3.8 MB)]
Example 8
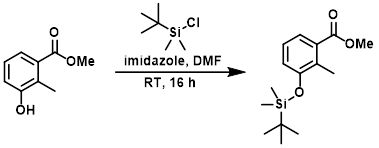
To a 1L, 3-neck, RBF equipped with a stirring bar, thermometer, and ice bath, was added DMF (300 mL), the SM (90 g, 542 mmol), and imidazole (92 g, 1.354 mol). TBS-Cl (90 g, 596 mmol) was added to the above solution in portions to control the internal temp between 15-19 C over 20 min. After the addition, the internal temp dropped below 1 C. The ice bath was removed and the reaction mixture was stirred at RT for 16 h. The reaction mixture was added to ice-H2O (500 mL), and the resulting solution was divided into two portions (2 x 700 mL). Each portion was extracted with EtOAc (700 mL). Each organic layer was washed with cold H2O (350 mL) and brine (350 mL). The combined organics were dried (MgSO4) and concentrated to provide the product as a light-brown oil which was used in the next step without further purification. [160 g, 100% crude yield]
[Patent Reference: WO2014025960, page 95, ![]() (8.1 MB)]
(8.1 MB)]
