SNAr (Cl)
[Aliphatic Amines (2o)]
Examples:
Example 1
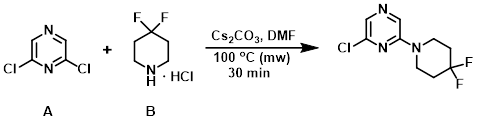
A mixture of the amine (B) (598 mg, 3.79 mmol), Cs2CO3 (2.47 g, 7.58 mmol), and the aryl chloride (A) (538 mg, 3.61 mmol) in DMF (5 mL) was irradiated in a microwave reactor at 100 C for 30 min. The mixture was diluted with EtOAc (50 mL) and washed with H2O (2 x 5 mL). The org layer was dried, concentrated, and purified by silica gel chromatography (25-35% EtOAc/hexane) to provide the product as a brown amorphous solid. [717 mg, 85%]
[Patent Reference: WO2012129338, page 70, ![]() (12.0 MB)]
(12.0 MB)]
Example 2
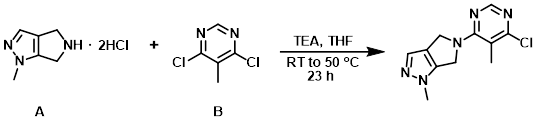
The amine (A) (2.00 g, 10.2 mmol) and the aryl chloride (B) (1.66 g, 10.2 mmol) were suspended in THF (51 mL) at RT. To this mixture was added TEA (4.41 mL, 31.6 mmol), leading to cloudiness in the mixture and a brown solid sticking to the flask walls. The reaction mixture was stirred at RT for 4 h, then heated at 50 C for 19 h. The mixture was cooled to RT and diluted with H2O (100 mL). The mixture was extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the product as a light brown solid. [1.95 g, 78%]
[Patent Reference: WO2012069948, page 84, ![]() (3.9 MB)]
(3.9 MB)]
Example 3
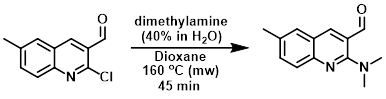
To a stirred solution of the SM (1.0 g, 4.86 mmol) in dioxane (10 mL) in a 20 mL microwave vial was added dimethylamine (40% in H2O, 6.1 mL, 48.6 mmol). The resulting mixture was irradiated at 160 C in a microwave reactor for 45 min, after which time it was concentrated in vacuo. The resulting crude was dissolved in DCM (30 mL), washed with H2O (3 x 20 mL), brine (20 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as a yellow oil. [988 mg, 95%]
[Patent Reference: WO2012112946, page 113, ![]() (11.2 MB)]
(11.2 MB)]
Example 4
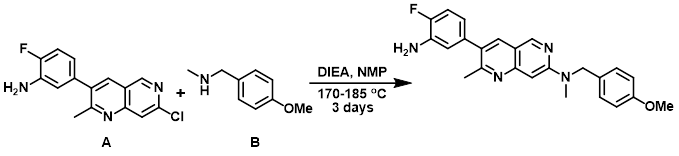
To a solution of the aryl chloride (A) (353 mg, 1.227 mmol) and the amine (B) (371 mg, 2.454 mmol) in NMP (6 mL) in a sealed tube was added DIEA (0.429 mL, 2.454 mmol). The mixture was sparged with argon and stirred at 170 C overnight. Additional amine (371 mg, 2.454 mmol) was added and the mixture was stirred at 185 C for 24 h. Additional amine (150 mg, 1.0 mmol) was again added and the mixture was stirred at 185 C overnight. The mixture was cooled to RT, diluted with EtOAc, and washed with sat aq NaHCO3, H2O, and brine. The org layer was dried (MgSO4), concentrated, and purified by silica gel chromatography (EtOAc/hexane) to provide the product. [334 mg, 68%]
[Patent Reference: WO2013134298, page 48, ![]() (4.1 MB)]
(4.1 MB)]
Example 5
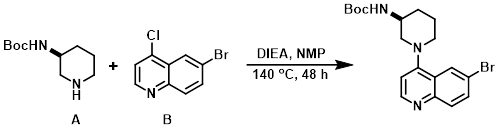
A solution of the aryl chloride (B) (1.0 equiv), the amine (A) (1.0 equiv), and DIEA (1.5 equiv) in NMP (0.1 M) was heated at 140 C for 48 h. Upon cooling the solution was poured onto ice and upon melting of the ice the solid was filtered, rinsed with H2O, and dried to provide the product.
[Patent Reference: WO2010026121, page 52, ![]() (3.6 MB)]
(3.6 MB)]
