PMB Deprotection
(TFA)
Examples:
Example 1

A solution of the SM (70 mg, 0.19 mmol) in TFA (2 mL) was stirred at 80 C for 16 h. After concentration, the residue was dissolved in EtOAc (10 mL) and washed with sat aq NaHCO3 (10 mL), brine (10 mL), dried (MgSO4), and concentrated in vacuo. The resulting crude material was purified by Prep HPLC (5-95% MeOH/H2O with 0.05% NH4OH) to provide the product as a white solid. [40 mg, 31%]
[Patent Reference: WO2016011390, page 105, ![]() (20.2 MB)]
(20.2 MB)]
Example 2

A mixture of the SM (21 g, 65.6 mmol) in TFA (150 mL) was stirred at 50 C overnight. The mixture was cooled to RT and concentrated. The resulting material was dissolved in EtOAc, washed with sat aq NaHCO3 (2x), brine (1x), dried (MgSO4), concentrated, and purified by silica gel chromatography to provide the product. [10 g, 76%]
[Patent Reference: WO2013134298, page 39, ![]() (4.1 MB)]
(4.1 MB)]
Example 3
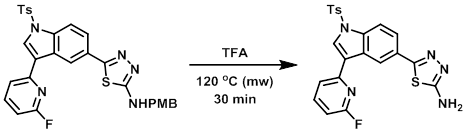
A glass microwave vessel was charged with the SM (400 mg, 0.683 mmol) and TFA (0.5 mL). The reaction was stirred at 120 C in a microwave reactor for 30 min. The solvent was removed in vacuo and the resulting residue was diluted with DCM and washed with sat aq NaHCO3. The org layer was dried and concentrated. The resulting material was purified by silica gel chromatography (10-25% EtOAc/DCM with 1% MeOH) to provide the product as a white solid. [250 mg, 79%]
[Patent Reference: WO2012129338, page 100, ![]() (12.0 MB)]
(12.0 MB)]
Example 4
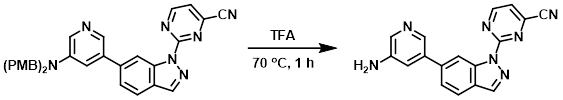
A solution of the SM (80 mg, 0.267 mmol) in TFA (5 mL) was stirred at 70 C for 1 h. After concentration, the residue was purified by Prep HPLC [5-95% (ACN)/(0.5% TFA in H2O)] to provide the product as a yellow oil. [14 mg, 31%]
[Patent Reference: WO2016011390, page 85, ![]() (20.2 MB)]
(20.2 MB)]
Example 5
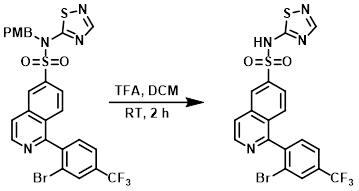
To a solution of the SM (100 mg, 0.157 mmol) in DCM (1.57 mL) was added TFA (60.6 uL, 0.787 mmol). The reaction mixture was stirred at RT for 2 h, after which time LCMS indicated mostly desired product. The mixture was concentrated and dissolved in DMSO (3 mL). This solution was injected onto a reverse phase HPLC (Xbridge 10 uM, C18, 19x100 mm column eluting with 0.1% NH4OH in ACN and H2O as the mobile phase) to provide the product. [10.6 mg]
[Patent Reference: WO2014201173, page 55, ![]() (19.7 MB)]
(19.7 MB)]
Example 6

CF3SO3H (119 mL, 1.350 mol) was added to a mixture of the SM (178 g, 450 mmol) in TFA (750 mL). The reaction mixture was stirred at RT overnight. The solvent was removed in vacuo and the resulting residue was used directly in the next step. [78.4 g]
[Patent Reference: WO2015158653, page 28, ![]() (2.9 MB)]
(2.9 MB)]
