PMB Protection
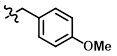
Common Conditions (Protection):
PMB-Cl
p-methoxybenzyl protection is typically performed using p-methoxybenzyl chloride (PMBCl) in the presence of a base.[1][2]
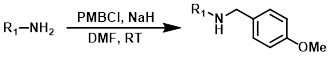
Common Conditions (Deprotection):
TFA
Trifluoroacetic acid (TFA) is commonly employed to deprotect PMB ethers. Other acid sensitive functionalities may not be well tolerated.[1][2]
References:
1) Kocienski, P. J.; Protecting Groups, 3rd Edition
2) Wuts, P. G. M.; Greene, T. W.; Greene's Protective Groups in Organic Synthesis, 4th Edition
