Boc Deprotection
(HCl)
Examples:
Example 1
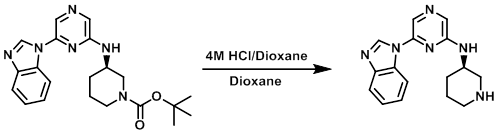
The SM (80 mg) was dissolved in dioxane (5 mL) and treated with 4M HCl in dioxane (2 mL). A yellow precipitate formed after 2 h. The solvent was removed in vacuo and the resultant yellow solid was dissolved in MeOH (1 mL). The solution was passed through an SCX column and eluted with 4N NH3 in MeOH. The methanolic solution was concentrated under reduced pressure to provide the product as a colorless oil. [57 mg, 48%]
[Patent Reference: WO2010016005, page 81, ![]() (11.3 MB)]
(11.3 MB)]
Example 2

A mixture of SM (89 mg) and 4N HCl in dioxane (2 mL) was stirred at RT for 2 h. The solvent was removed in vacuo and the resulting yellow solid was triturated with EtOAc to provide the bis-HCl product as a yellow solid. [75 mg, 91%]
[Patent Reference: WO2010016005, page 82, ![]() (11.3 MB)]
(11.3 MB)]
Example 3
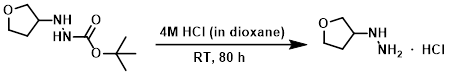
The SM (8.14 g, 40 mmol) was stirred in a solution of 4M HCl in dioxane (60 mL) at RT for 16 h. Additional 4M HCl in dioxane (20 mL) was added and the mixture was stirred at RT for another 64 h, after which time it was concentrated in vacuo to provide the product as a solid. [5.60 g, 100%]
[Patent Reference: WO2010032200, page 138, ![]() (6.2 MB)]
(6.2 MB)]
Example 4

To a stirred solution of the SM (500 mg, 1.30 mmol) in dioxane (10 mL) under N2 was added 4N HCl in dioxane (3 mL) dropwise. The reaction mixture was stirred at RT for 24 h, after which time the mixture was concentrated to give the product (white solid) as the HCl salt. [320 mg]
[Patent Reference: WO2010038081, page 115, ![]() (33.8 MB)]
(33.8 MB)]
Example 5
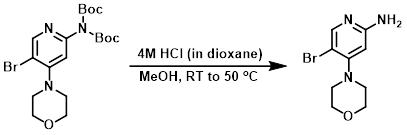
The SM (1.632 g, 3.56 mmol) was dissolved in dry MeOH (10 mL) and 4M HCl in dioxane (2.67 mL, 10.68 mmol) was added. The mixture was allowed to stir at RT overnight, then was heated at 50 C for 7 h. The reaction mixture was concentrated to provide the crude product which was used in the next step without further purification. [926 mg]
[Patent Reference: WO2010038081, page 276, ![]() (33.8 MB)]
(33.8 MB)]
Example 6

To a suspension of the SM (4.65 g, 9.05 mmol) in MeOH (30 mL) was added concentrated HCl (6.74 mL) and H2O (14 mL). The mixture was stirred at RT overnight, after which time 50% NaOH in H2O (4.8 mL) was added at 0 C to pH 9. The resulting yellow solids were filtered, rinsed with H2O (25 mL), and air dried for 3 days to provide the product. [3.75 g, 100%]
[Patent Reference: WO2012129344, page 127, ![]() (7.3 MB)]
(7.3 MB)]
Example 7
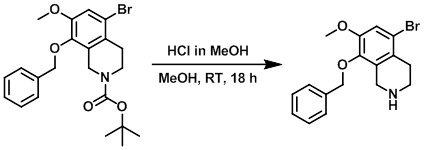
To a solution of the SM (25 g, 43 mmol) in MeOH (200 mL) was added a solution of HCl in MeOH (50 mL). The reaction mixture was stirred at RT for 18 h, then adjusted to pH 8-9 with 2 N NaOH. The mixture was extracted with DCM (2 x 500 mL). The combined organics were washed with sat aq brine (3 x 300 mL), dried (Na2SO4), and concentrated in vacuo, to provide the product as a white solid. [10.3 g, 29.6 mmol, 69% over 2 steps]
[Patent Reference: WO2014177977, page 65, ![]() (6.0 MB)]
(6.0 MB)]
Example 8

To a solution of the SM (910 mg, 3.44 mmol) in MeOH/EtOAc (1:1, 5 mL) was added conc. HCl (5 drops). After 3 h, the reaction mixture was concentrated in vacuo to provide the product as a solid. [700 mg, quantitative]
[Patent Reference: WO2014177977, page 68, ![]() (6.0 MB)]
(6.0 MB)]
Example 9
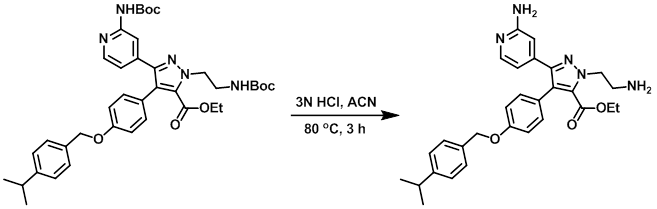
A solution of the SM (0.75 g, 1.1 mmol) and 3N HCl (1.8 mL, 5.4 mmol) in ACN (19 mL) was stirred at 80 C for 3 h. The reaction mixture was concentrated. To the resulting material was added 10% aq K2CO3, and the mixture was extracted with DCM. The org layer was dried (MgSO4) and concentrated in vacuo to provide the product as a white solid. [0.49 g, 92%]
[Patent Reference: WO2015144799, page 101, ![]() (18.8 MB)]
(18.8 MB)]
