Alcohol to Chloride
Sulfonyl Chlorides (R-SO2Cl)
Examples:
Example 1
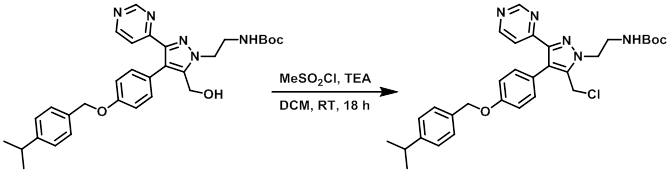
To a stirred solution of the SM (1.66 g, 3.05 mmol) in dry DCM (35 mL) at 0 C was added TEA (0.679 mL, 4.89 mmol) and MeSO2Cl (0.354 mL, 4.58 mmol). The reaction mixture was stirred at RT for 18 h, after which time was added sat aq NaHCO3 and DCM. The org layer was separated, washed with brine, dried (MgSO4), and concentrated in vacuo to provide the product as a brown solid. [1.76 g, quant.]
[Patent Reference: WO2015144799, page 325, ![]() (18.8 MB)]
(18.8 MB)]
Example 2
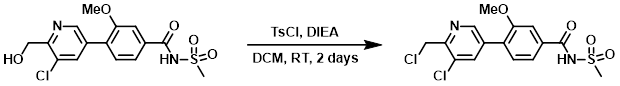
To a 50 mL RBF was added the SM (612 mg, 1.65 mmol) in DCM (8.25 mL). DIEA (0.574 mL, 3.30 mmol) was added, followed by TsCl (378 mg, 1.98 mmol). The reaction mixture was stirred at RT for 2 days. The crude material was adsorbed onto a plug of silica gel and was purified by silica gel column chromatography (12 g silica, 0-10% MeOH/DCM) to provide the product. [400 mg, 62%]
[Patent Reference: WO2015051043, page 85, ![]() (9.7 MB)]
(9.7 MB)]
