SNAr (Cl)
(N-Heteroaryls)
Examples:
Example 1
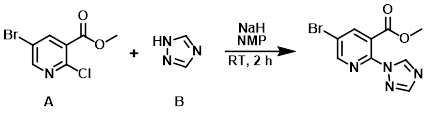
The triazole (B) (0.4 g, 5.79 mmol) in NMP (5 mL) was treated with NaH (60% dispersion in oil, 200 mg, 5.00 mmol). The mixture was stirred at RT for 30 min, after which time the aryl chloride (A) (1 g, 3.99 mmol) was added. The mixture was stirred for 2 h, diluted with H2O, extracted with EtOAc, washed with brine, dried (MgSO4), and concentrated. The crude was purified by flash chromatography (20-60% EtOAc/hexane) to provide the product as an off-white solid. [0.42 g]
[Patent Reference: WO2010038081, page 148, ![]() (33.8 MB)]
(33.8 MB)]
Example 2
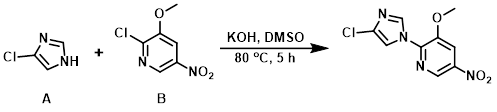
A mixture of the aryl chloride (B) (5.0 g, 26.5 mmol), the imidazole (A) (2.72 g, 26.5 mmol), and KOH flakes (1.488 g, 26.5 mmol) in dry DMSO (25 mL) was heated at 80 C for 5 h. The reaction mixture was allowed to cool to RT and was poured into 1.0 L of H2O with vigorous stirring. The mixture was stirred at RT for 16 h, after which time the precipitate was collected by vacuum filtration. The solid was dried under vacuum for 24 h to provide the product as a light brown solid. [5.22 g, 77%]
[Patent Reference: WO2011014535, page 47, ![]() (17.3 MB)]
(17.3 MB)]
Example 3
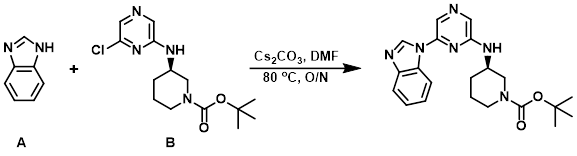
Benzimidazole (A) (76 mg, 0.64 mmol), the aryl chloride (B) (200 mg, 0.639 mmol), and Cs2CO3 (312 mg, 0.959 mmol) were dissolved in DMF (2 mL). The mixture was heated to 80 C overnight. The reaction mixture was diluted with H2O (25 mL) and extracted with DCM (2 x 20 mL). The org layer was concentrated in vacuo and the crude product was purified by silica gel flash chromatography (0-45% EtOH/EtOAc) to provide the product as a clear oil. [80 mg, 32%]
[Patent Reference: WO2010016005, page 80, ![]() (11.3 MB)]
(11.3 MB)]
