Iodination
(Lithiation / Iodination)
Examples:
Example 1
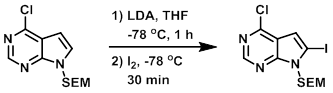
To a solution of the SM (5.56 g, 19.6 mmol) in THF (20 mL) was added LDA (2M in THF/heptane/ethylbenzene, 19.6 mL, 39.2 mmol) dropwise at -78 C. The reaction was stirred at -78 C for 1 h, after which time was added dropwise a solution of Iodine (9.94 g, 39.2 mmol) in THF (10 mL). After 30 min, the dry ice bath was removed and the reaction was warmed to RT. The mixture was quenched by the addition of sat aq Na2S2O3, then extracted with EtOAc. The combined organics were washed with H2O, brine, dried (MgSO4), and concentrated. The resulting material was purified by silica gel chromatography (0-20% EtOAc/hexane) to provide the product as a white solid. [2.65 g, 33%]
[Patent Reference: WO2012149280, page 76, ![]() (4.1 MB)]
(4.1 MB)]
Example 2
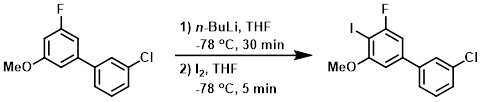
A RBF was charged with the SM (577.6 mg, 2.441 mmol) and THF (8.1 mL) to give a clear solution. The flask was cooled in a dry ice/acetone bath for 10 min, the treated dropwise with n-BuLi (1.8M in hexanes, 1.627 mL, 2.93 mmol). After 30 min, a solution of Iodine (929 mg, 3.66 mmol) in THF (3 mL) was added dropwise. TLC after 5 min showed conversion to a slightly lower spot. The mixture was diluted with sat aq Na2S2O3 and warmed to RT. The mixture was diluted with H2O and extracted with EtOAc (2x). The combined organics were dried (Na2SO4) and concentrated. The residue was purified by silica gel column chromatography (25 g Ultra SNAP column, 0-5% EtOAc/heptane) to provide the product as an off-white solid. [687 mg, 78%]
[Patent Reference: WO2014201173, page 366, ![]() (19.7 MB)]
(19.7 MB)]
Example 3
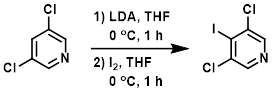
To a stirring solution of the SM (3.0 g, 20.4 mmol) in THF (15 mL) was added LDA (2.0 M in THF/heptane/ethylbenzene, 12.14 mL, 24.4 mmol) dropwise at 0 C. The mixture was stirred at 0 C for 1 h. A solution of Iodine (2.7 g, 21.4 mmol) in THF (10 mL) was added dropwise. Upon completion of the addition, the reaction mixture was stirred at 0 C another 1 h. The mixture was quenched with H2O (40 mL) and extracted with EtOAc (4 x 50 mL). The combined organics were washed with brine (50 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as a yellow gum. [3.2 g, 57%]
[Patent Reference: WO2015129926, page 152, ![]() (21.5 MB)]
(21.5 MB)]
