Alcohol to Triflate
(Tf2O)
Examples:
Example 1

To a solution of the SM (100 g, 380 mmol) and TEA (76.8 g, 760 mmol) in DCM (1.5 L) at 0 C was added Tf2O (107 g, 380 mmol) via addition funnel. Upon completion of the Tf2O addition, the solution was warmed to RT for 5 h. The mixture was then treated with H2O and DCM. The layers were separated and the org layer was washed with brine, dried (Na2SO4), and concentrated. The residue was purified by silica gel flash chromatography (20:1 PE/EtOAc) to provide the product. [105 g, 70%]
Example 2
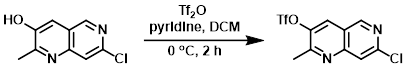
To a solution of the SM (30 g, 155 mmol) in DCM (300 mL) at 0 C under argon was added pyridine (24.4 g, 309 mmol) and Tf2O (65.4 g, 232 mmol). The reaction mixture was stirred for 2 h. The mixture was washed with H2O and the layers were separated. The aq layer was further extracted with DCM (1x). The combined organics were washed with brine, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel chromatography to provide the product. [40.2 g, 80%]
[Patent Reference: WO2013134298, page 45, ![]() (4.1 MB)]
(4.1 MB)]
Example 3
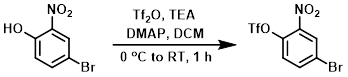
To a solution of the SM (5.0 g, 22.9 mmol) in DCM (100 mL) at 0 C was added TEA (2.8 g, 27.5 mmol), DMAP (280 mg, 2.3 mmol), and Tf2O (7.8 g, 27.5 mmol). The reaction mixture was stirred at RT for 1 h. After completion of the reaction, the mixture was partitioned between DCM and H2O. The org layer was washed with sat aq NaHCO3, brine, dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography to provide the product as a yellow oily liquid. [7.2 g, 90%]
[Patent Reference: WO2014149164, page 429, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
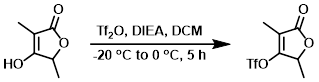
Tf2O (23.7 mL, 140 mL) was added portionwise to a solution of the SM (15.0 g, 117 mmol) and DIEA (24.8 mL, 140 mmol) in DCM (500 mL) at -20 C, at a rate sufficient to maintain the internal temp below -10 C. The reaction mixture was allowed to warm gradually from -20 C to 0 C over 5 h. The mixture was then passed through a silica gel plug, dried (MgSO4), and concentrated in vacuo. The residue was suspended in ether and filtered. The filtrate was concentrated and purified by silica gel chromatography (0-17% EtOAc/heptane) to provide the product as a pale yellow oil. [21.06 g, 69%]
