Sodium Borohydride
Other Names:
Sodium tetrahydridoborate
Sodium tetrahydroborate
General Information:
Structure:

CAS Number: 16940-66-2
Molecular Weight: 37.83 g/mol
Appearance: White solid
Sodium borohydride (NaBH4) is a mild reducing agent that is typically used to reduce aldehydes and ketones to their respective alcohols. NaBH4 is sometimes used to reduce esters to alcohols but the reaction is generally slow. This difference in reactivity usually allows for the selective reduction of aldehydes and ketones in the presence of esters. Carboxylic acids, nitriles, and amides are not reduced by NaBH4 under normal conditions. However, carboxylic acids can be reduced by NaBH4 if they have been activated by an appropriate activating agent. NaBH4 is also occasionally used for reductive aminations.
NaBH4 is usually used in hydroxylic solvents such as MeOH, EtOH, and H2O. Sometimes THF is used as a solvent, either alone or as a solvent mixture (ex. THF/MeOH or THF/EtOH). In MeOH and EtOH, NaBH4 decomposes over time to give the respective borates. An excess of NaBH4 can be used to compensate for the decomposition of the reagent over time.
Common Uses:
Reagent for the reduction of aldehydes to alcohols

Procedure excerpt:
The SM (785 mg, 2.71 mmol) was dissolved in THF (5.0 mL) and MeOH (5.0 mL). The mixture was cooled to 0 C and treated with NaBH4 (156 mg, 4.07 mmol). The reaction . . .
Reagent for the reduction of ketones to alcohols
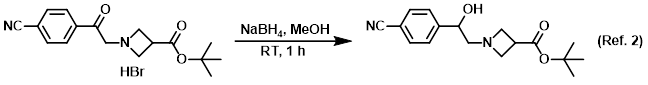
Procedure excerpt:
To a mixture of the SM (170 mg, 0.446 mmol) in MeOH (5 mL) was added NaBH4 (25 mg, 0.661 mmol). The reaction mixture was stirred at RT for 1 h, after which time . . .
Reagent for the reduction of esters to alcohols
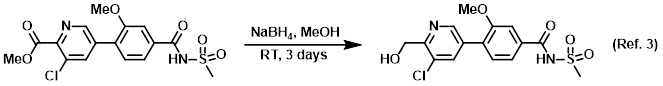
Procedure excerpt:
NaBH4 (201 mg, 5.32 mmol) was added slowly into a solution of the SM (530 mg, 1.33 mmol) in MeOH (4.43 mL) at RT. The reaction mixture was stirred for 2 days. . . .
Reagent for the reduction of activated carboxylic acid derivatives to alcohols
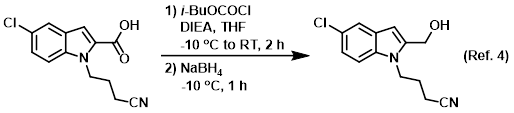
Procedure excerpt:
. . . a solution of isobutylchloroformate in THF (50 mL) was added dropwise and stirring was continued for 1 h at -10 C, then 1 h at RT. NaBH4 (17.02 g, 450 mmol) was added . . .
Reagent for reductive amination
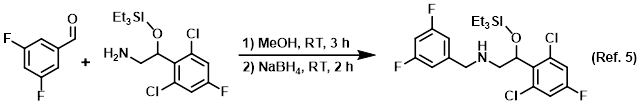
Procedure excerpt:
. . . stirred at RT for 2 h. Upon completion of imine formation (monitored by TLC), NaBH4 (4.9 g, 133.1 mmol) was added portionwise at 0 C. The mixture was warmed to RT . . .
Safety:
Sodium borohydride (NaBH4) decomposes rapidly in water (especially if acidic). The reaction is exothermic and produces diborane gas (toxic), hydrogen gas (flammable), and boric acid (weak acid).
References:
1) Patent Reference: WO2016014463, page 99, ![]() (6.7 MB)
(6.7 MB)
2) Patent Reference: WO2011017578, page 127, ![]() (8.3 MB)
(8.3 MB)
3) Patent Reference: WO2015051043, page 85, ![]() (9.7 MB)
(9.7 MB)
4) Patent Reference: WO2015158653, page 35, ![]() (2.9 MB)
(2.9 MB)
5) Patent Reference: WO2015129926, page 74, ![]() (21.5 MB)
(21.5 MB)
6) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
7) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
8) Wikipedia: Sodium borohydride (link)
9) www.sigmaaldrich.com: Sodium borohydride (link)
