Ester to Alcohol
(NaBH4)
Examples:
Example 1
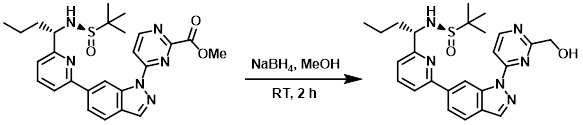
To a suspension of the SM (500 mg, 0.99 mmol) in MeOH (50 mL) at 0 C was added NaBH4 (113 mg, 2.96 mmol). The reaction mixture was stirred at RT for 2 h. After concentration, the residue was diluted with H2O (100 mL) and extracted with EtOAc (3 x 30 mL). The combined organics were washed with brine (30 mL), dried (Na2SO4), and concentrated. The resulting material was purified by Prep TLC (1:20 MeOH/DCM) to provide the product as a white solid. [300 mg, 64%]
[Patent Reference: WO2016011390, page 321, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
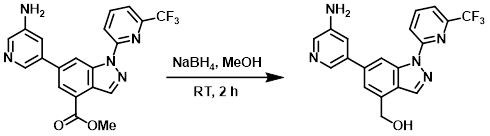
To a solution of the SM (50 mg, 0.12 mmol) in MeOH (5 mL) was added NaBH4 (9.0 mg, 0.24 mmol) at RT. The reaction mixture was stirred at RT for 2 h. After concentration, the residue was purified by Prep HPLC [5-95% (MeOH)/(H2O with 0.05% TFA)] to provide the product as a yellow solid. [20 mg, 52%]
[Patent Reference: WO2016011390, page 103, ![]() (20.2 MB)]
(20.2 MB)]
Example 3
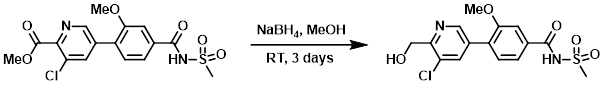
NaBH4 (201 mg, 5.32 mmol) was added slowly into a solution of the SM (530 mg, 1.33 mmol) in MeOH (4.43 mL) at RT. The reaction mixture was stirred for 2 days. Another portion of NaBH4 (201 mg, 5.32 mmol) was added and the reaction was stirred another day. The reaction mixture was concentrated in vacuo. The residue was dissolved in DCM and washed with H2O. The combined aq layers were concentrated in vacuo. The resulting crude material was adsorbed onto a plug of silica gel and purified by silica gel column chromatography (12 g silica, 0-10% MeOH/DCM) to provide the product as an off-white solid. [80 mg, 16%]
[Patent Reference: WO2015051043, page 85, ![]() (9.7 MB)]
(9.7 MB)]
Example 4
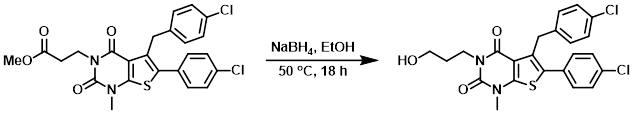
To a solution of the SM (13 mg, 0.0258 mmol) in EtOH (1 mL) was added NaBH4 (2.9 mg, 0.0775 mmol). The reaction was stirred at 50 C for 18 h. The mixture was cooled to RT, quenched with aq 0.1N HCl (2 mL), and concentrated in vacuo. The resulting residue was purified by Prep HPLC to provide the product as a white solid. [8.6 mg, 70%]
[Patent Reference: WO2016023832, page 67, ![]() (3.2 MB)]
(3.2 MB)]
Example 5
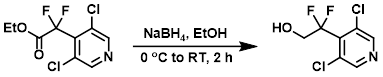
To a stirring solution of the SM (315 mg, 1.16 mmol) in EtOH (10 mL) was added solid NaBH4 (16.2 mg, 1.74 mmol) in portions at 0 C. The reaction mixture was warmed to RT and stirred for 2 h. The mixture was quenched with H2O (30 mL) and extracted with EtOAc (3 x 30 mL). The combined organics were washed with brine (2 x 30 mL), dried (Na2SO4), and concentrated. The residue was purified by silica gel column chromatography (55% EtOAc/hexane) to provide the product as a colorless gum. [180 mg, 44%]
[Patent Reference: WO2015129926, page 152, ![]() (21.5 MB)]
(21.5 MB)]
