Urea Formation
(Amine + Carbamate)
Examples:
Example 1
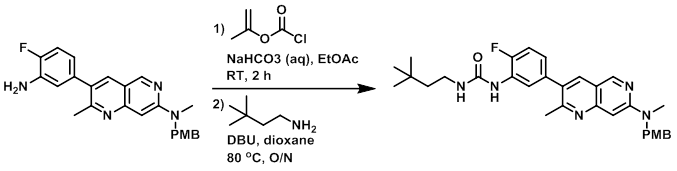
Step 1:
To a solution of the SM (334 mg, 0.830 mmol) in EtOAc (10 mL) was added sat aq NaHCO3 (10 mL), followed by isopropenyl chloroformate (0.100 mL, 0.913 mmol). The reaction mixture was stirred at RT for 2 h. The layers were separated and the aq layer was further extracted with EtOAc (2x). The combined organics were dried (MgSO4) and concentrated to provide the isopropenyl carbamate intermediate. [398 mg, 99%]
[Patent Reference: WO2013134298, page 56, ![]() (4.1 MB)]
(4.1 MB)]
Step 2:
To a solution of the isopropenyl carbamate (398 mg, 0.818 mmol) and 3,3-dimethylbutylamine (166 mg, 1.636 mmol) in dioxane (10 mL) was added DBU (0.025 mL, 0.164 mmol). The reaction was stirred at 80 C overnight. The mixture was cooled to RT and diluted with EtOAc. The mixture was washed with 10% LiCl, then brine. The org layer was dried (MgSO4), concentrated, and purified by silica gel chromatography (EtOAc/hexane) to provide the product. [370 mg, 85%]
[Patent Reference: WO2013134298, page 81, ![]() (4.1 MB)]
(4.1 MB)]
Example 2
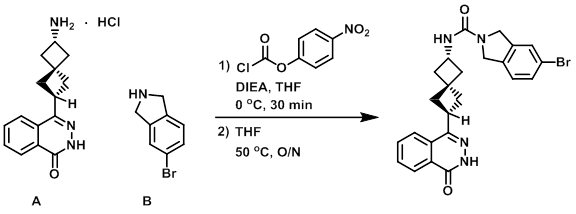
A (300 mg, 1.028 mmol) was suspended in THF (15 mL), and DIEA (0.449 mL, 2.57 mmol) was added. The mixture was cooled to 0 C, and 4-nitrophenyl chloroformate (249 mg, 1.23 mmol) was added. The mixture was stirred at 0 C for 30 min, after which time was added B (407 mg, 2.06 mmol) and DIEA (0.449 mL, 2.57 mmol). The reaction mixture was stirred at 50 C for 16 h. The mixture was cooled to RT, quenched with MeOH (3 mL), and concentrated. The resulting material was purified by flash chromatography (30-100% EtOAc/DCM) to provide the product as a light yellow solid. [400 mg, 81%]
[Patent Reference: WO2016010950, page 214, ![]() (18.8 MB)]
(18.8 MB)]
