O-Demethylation
(BBr3)
Examples:
Example 1
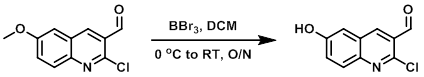
A mixture of the SM (1.01 g, 4.54 mmol) in dry DCM (36 mL) at 0 C under N2 was treated dropwise with BBr3 (1M in DCM, 13.6 mL, 13.6 mmol). The resulting mixture was allowed to warm to RT and stir overnight, after which time it was added dropwise to a stirring mixture of ice water (50 mL). The mixture was stirred for 30 min at RT then filtered and dried to provide the product as a yellow solid. [0.77 g, 82%]
[Patent Reference: WO2012112946, page 128, ![]() (11.2 MB)]
(11.2 MB)]
Example 2
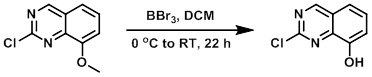
To a 0.55M solution of the SM in DCM at 0 C was added BBr3 (1.0M in DCM, 2.2 equiv) over 5 min. The reaction mixture was stirred at RT for 22 h, and then cooled to -5 C for 30 min. The solids were filtered and then stirred in ice/H2O for 30 min. The solids were filtered, rinsed with IPA, and dried in a desiccator to provide the product. [79%]
[Patent Reference: WO2007117607, page 305, ![]() (12.9 MB)]
(12.9 MB)]
Example 3
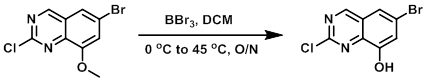
To a suspension of the SM (1.26 g, 4.6 mmol) in DCM (20 mL) at 0 C was added dropwise BBr3 (1M in DCM , 28 mL, 28 mmol). The reaction was stirred at 45 C overnight. The mixture was concentrated to dryness, cooled in an ice bath, and diluted with sat aq NaHCO3. The resulting solids were collected and air dried to provide impure product which was used without further purification.
[Patent Reference: WO2007117607, page 314, ![]() (12.9 MB)]
(12.9 MB)]
Example 4
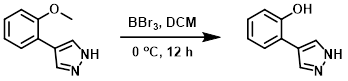
To a solution of the SM (260 mg, 1.5 mmol) in DCM (5 mL) was added BBr3 (750 mg, 3.0 mmol) at 0 C. The reaction mixture was stirred at 0 C for 12 h. The mixture was diluted with DCM (20 mL), washed with sat aq NaHCO3 (2 x 20 mL), brine, dried (Na2SO4), and concentrated to provide the product as a yellow oil. [200 mg, 84%]
[Patent Reference: WO2015140133, page 109, ![]() (11.7 MB)]
(11.7 MB)]
Example 5
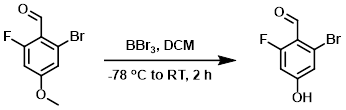
To a solution of the SM (21 g, 90 mmol) in DCM (100 mL) at -78 C was slowly added BBr3 (46 g, 180 mmol). The reaction mixture was stirred at RT for 2 h, after which time the mixture was quenched with H2O (50 mL). The mixture was filtered and the solids were washed with DCM (2 x 200 mL). The filtrate was washed with H2O (2 x 100 mL). The org layer was dried (Na2SO4) and concentrated to provide the product was a yellow oil. [13.0 g, 66%]
[Patent Reference: WO2016011390, page 282, ![]() (20.2 MB)]
(20.2 MB)]
Example 6
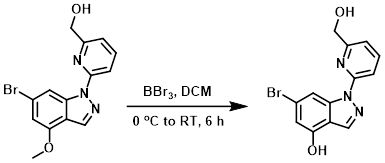
To a suspension of the SM (8.0 g, 24.0 mmol) in DCM (100 mL) at 0 C was slowly added BBr3 (3M in DCM, 24 mL, 72.0 mmol). The reaction mixture was strirred at RT for 6 h, after which time it was quenched with MeOH (50 mL). The solids were collected by filtration and washed with MeOH (2 x 5 mL) to provide the product as a yellow solid. [6.2 g, 80%]
[Patent Reference: WO2016011390, page 370, ![]() (20.2 MB)]
(20.2 MB)]
