Boron Tribromide
Other Names:
Tribromoboron
Tribromoborane
Boron bromide
General Information:
Structure:
![]()
CAS Number: 10294-33-4
Molecular Weight: 250.52 g/mol
Appearance: Colorless to amber liquid
Chemical Formula: BBr3
Melting Point: -46.3
Boiling Point: 91.3 C
Density: 2.60 g/mL
Boron tribromide (BBr3) is a Lewis acid commonly used for the demethylation of methyl ethers. Boron tribromide is available neat, but is often purchased as a 1M solution in either DCM, hexane, or heptane. The most common of these is the 1M solution in DCM. Boron tribromide is moisture sensitive so reactions should be carried out under anhydrous conditions. Ether and THF are not suitable solvents since BBr3 cleaves ethers.
Common Uses:
Reagent for the demethylation of methyl ethers
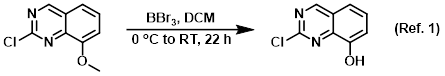
Procedure excerpt:
To a 0.55M solution of the SM in DCM at 0 C was added BBr3 (1.0M in DCM, 2.2 equiv) over 5 min. The reaction mixture was stirred at RT for 22 h, and . . .
Safety:
Boron tribromide (BBr3) reacts violently with water and other protic solvents. BBr3 decomposes when exposed to moisture in the air to evolve HBr. Always handle under inert atmosphere.
References:
1) Patent Reference: WO2007117607, page 305, ![]() (12.9 MB)
(12.9 MB)
2) www.sigmaaldrich.com - Boron tribromide (link)
3) Wikipedia: Boron tribromide (link)
4) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
