Hydrobromic Acid
General Information:
Structure:
![]()
CAS Number: 10035-10-6
Molecular Weight: 80.91 g/mol
Appearance: Colorless to light yellow liquid (48% w/w aq.)
Density: 1.49 g/mL (48% w/w aq.)
Hydrobromic acid (HBr) is commonly sold as a 48% (by weight) solution in water. HBr works well as a Lewis acid catalyst but the increased nucleophilicity of bromide relative to chloride generally makes hydrochloric acid the preferable choice. However, in certain reactions such as in the deprotection of methyl ethers, the increased nucleophilicity of bromide is advantageous. HBr is also a reasonable choice in reactions where the presence of bromide already exists, such as Sandmeyer reactions and brominations.
Common Uses:
Reagent/solvent for the deprotection of phenolic methyl ethers
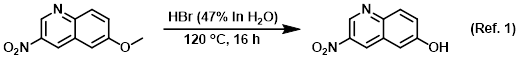
Procedure excerpt:
A mixture of the SM (100 mg, 0.49 mmol) in HBr (47% in H2O, 2.5 mL) was stirred at 120 C for 16 h. Upon completion, the reaction mixture was cooled to RT and . . .
Reagent/solvent in bromine-based Sandmeyer reactions
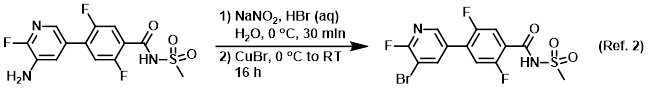
Procedure excerpt:
To a solution of the SM (8.00 g, 23.74 mmol) in HBr (48% in H2O, 150 mL) was added a solution of NaNO2 (16.3 g, 237 mmol) in H2O (50 mL), keeping the temp below 0 C. . . .
Reagent/solvent in bromination reactions
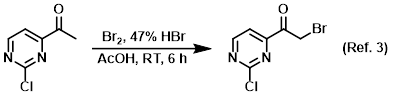
Procedure excerpt:
To a solution of the SM (1.0 g, 6.4 mmol) in AcOH (15 mL) was added Br2 (0.3 mL, 6.4 mmol) and 47% HBr (0.7 mL, 6.4 mmol). The resulting mixture was stirred at RT . . .
Safety:
Hydrobromic acid is very acidic. Contact with skin or eyes can cause severe burns.
References:
1) Patent Reference: WO2007084786, page 115, ![]() (9.4 MB)
(9.4 MB)
2) Patent Reference: WO2015051043, page 107, ![]() (9.7 MB)
(9.7 MB)
3) Patent Reference: WO2014149164, page 339, ![]() (23.7 MB)
(23.7 MB)
4) Wikipedia: Hydrobromic acid (link)
5) www.sigmaaldrich.com: Hydrobromic acid (link)
6) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
