Nitro Reduction
(H2 + Raney Ni)
Examples:
Example 1

To a shaker flask was added the SM (crude, 32.4 g, 138 mmol), EtOH (100 mL), and Raney Ni (1.00 g, 17.04 mmol). The flask was charged with H2 (275 kPa) and was agitated until the absorption of H2 ceased. The vessel was depressurized and the catalyst was removed via filtration. The filtrate was concentrated to dryness, diluted with MTBE, and filtered again. The filtrate was concentrated and the resulting residue was stirred in hexane. The solids were filtered, washed with cold hexane, and dried in vacuo to provide the product as a dark solid. [17.8 g, 63%]
[Patent Reference: WO2013134298, page 42, ![]() (4.1 MB)]
(4.1 MB)]
Example 2
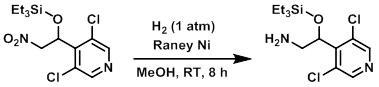
The SM (4.1 g, 11.6 mmol) and Raney Ni 2800 (690 mg, in H2O) in MeOH (50 mL) was hydrogenated under an atmosphere of H2 (1 atm) at RT for 8 h. The reaction mixture was filtered through a pad of celite and washed with EtOAc. The filtrate was concentrated in vacuo and the resulting residue was purified by silica gel column chromatography to provide the product as a white solid. [1.9 g, 50%]
[Patent Reference: WO2015129926, page 70, ![]() (21.5 MB)]
(21.5 MB)]
Example 3
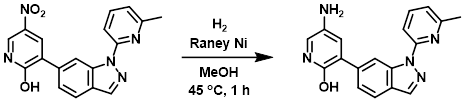
To a solution of the SM (50 mg, 0.14 mmol) in MeOH (5 mL) was added Raney Ni (5 mg, 10% w/w). The reaction mixture was stirred under H2 at 45 C for 1 h. After filtration, the filtrate was concentrated and purified by Prep HPLC [5-95% (MeOH)/(H2O with 0.05% NH4OH)] to provide the product as a yellow solid. [16 mg, 35%]
[Patent Reference: WO2016011390, page 62, ![]() (20.2 MB)]
(20.2 MB)]
