Mitsunobu
(Amides & Amide-like NH's)
Examples:
Example 1

DtBAD (99 mg, 0.43 mmol) was added portionwise to a solution of A (63 mg, 0.43 mmol), B (100 mg, 0.29 mmol), PPh3 supp. (134 mg, 0.43 mmol) in THF (3.3 mL) at RT under N2. The reaction mixture was stirred for 3 days. The mixture was filtered through a pad of celite and the pad was washed with EtOAc. The filtrate was concentrated and the resulting material was purified by Prep LC (12 g silica, 10-15% EtOAc/heptane) to provide the product. [63 mg, 46%]
[Patent Reference: WO2015144799, page 179, ![]() (18.8 MB)]
(18.8 MB)]
Example 2
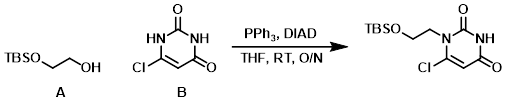
To a solution of B (100.0 g, 0.680 mol), A (143.6 g, 0.816 mol), and PPh3 (267.2 g, 1.02 mol) in dry THF (2.0 L) under N2 at RT was added dropwise DIAD (206.0 g, 1.02 mol) in dry THF (500 mL). The reaction mixture was stirred at RT overnight, after which time the mixture was concentrated in vacuo. The residue was triturated with PE/EtOAc (1:1, 500 mL), filtered, and concentrated. The residue was purified by silica gel column chromatography (5:1 PE/EtOAc) to provide the product as a white solid. [125.0 g, 60%]
[Patent Reference: WO2016011930, page 107, ![]() (15.7 MB)]
(15.7 MB)]
