Acid to Ester
(TMS-CHN2)
Examples:
Example 1

Trimethylsilyldiazomethane (2.0M in hexane, 25 mL, 50 mmol) was added dropwise to a 0 C solution of the SM (4.64 g, 41.4 mmol) in DCM (25 mL) and MeOH (5 mL). The reaction mixture was stirred at RT for 30 min. The mixture was quenched with AcOH (0.45 mL) and concentrated in vacuo. The resulting material was purified by silica gel chromatography (20% DCM/hexane) to provide the product as a colorless oil. [3.8 g, 73%]
[Patent Reference: WO2015129926, page 108, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
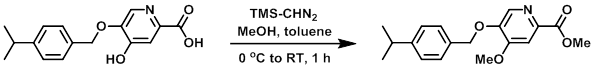
Trimethylsilyldiazomethane (2M in hexane, 10.4 mL, 20.8 mmol) was added to a solution of the SM (1.05 g, 3.66 mmol) in MeOH (5 mL) and toluene (20 mL) at 0 C under N2. The reaction mixture was stirred warming to RT for 1 h, after which time was added H2O and EtOAc. The layers were separated and the org layer was washed with brine, dried (MgSO4), and concentrated to give 830 mg of a brown solid. The material was combined with a separate batch of material (400 mg) and was purified by Prep LC (45 g silica, 10-75% EtOAc/heptane) to provide the product. [370 mg, 22% (global yield)]
[Patent Reference: WO2015144799, page 159, ![]() (18.8 MB)]
(18.8 MB)]
Example 3
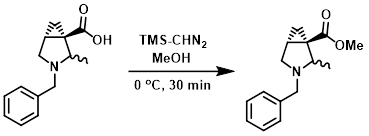
To a solution of the SM (2.6 g, 11 mmol) in MeOH (50 mL) at 0 C was added TMS-CHN2 until a yellowish color persisted. The mixture was stirred at 0 C for 30 min, after which time excess reagents were consumed by addition of AcOH. The mixture was concentrated in vacuo and the residue was purified by silica gel flash chromatography to provide the product. [1.8 g, 65%]
[Patent Reference: WO2016014463, page 68, ![]() (6.7 MB)]
(6.7 MB)]
