DIBAL-H
(Diisobutylaluminum hydride)
Other Names:
DIBALH
DIBAL
General Information:
Structure:
![]()
CAS Number: 1191-15-7
Molecular Weight: 142.22 g/mol
Appearance: Colorless liquid
Diisobutylaluminum hydride (DIBAL-H) is an organoaluminum reagent typically used for the reduction esters or nitriles to aldehydes. DIBAL-H is usually obtained and used as a 1.0 M solution in a wide variety of solvents (ex. DCM, THF, hexanes, heptane, toluene, and cyclohexane). Some of the advantages of using DIBAL-H over other organoaluminum reagents include its greater selectivity, its ease of handing as a liquid, and its miscibility with a wide range of solvents (including hydrocarbons and aromatic solvents).
Common Uses:
Reagent for reducing esters to aldehydes
![]()
Reagent for reducing esters to alcohols
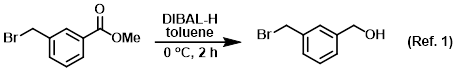
Procedure excerpt:
To a stirred solution of the SM (5 g, 21.8 mmol) in toluene (50 mL) was added DIBAL-H (43.6 mL, 43.6 mmol) at 0 C. The reaction mixture was stirred . . .
Reagent for reducing nitriles to aldehydes
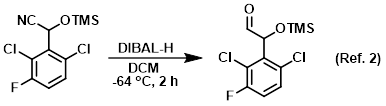
Procedure excerpt:
. . . purged with N2 and cooled to -64 C. Under N2 atmsophere, was added DIBAL-H (1.0M in hexane, 2.6 mL, 2.6 mmol). The mixture was stirred at -64 C for 2 h. While maintaining . . .
Reagent for reducing nitriles to amines
![]()
Safety:
Diisobutylaluminum hydride (DIBAL-H) reacts violently with water and moisture in the air (pyrophoric).
References:
1) Patent Reference: WO2015140133, page 106, ![]() (11.7 MB)
(11.7 MB)
2) Patent Reference: WO2015129926, page 111, ![]() (21.5 MB)
(21.5 MB)
3) www.akzonobel.com: Diisobutylaluminum hydride (DIBAL-H) and Other Isobutyl Aluminum Alkyls (DIBAL-BOT, TIBAL) as Specialty Organic Synthesis Reagents (link)
4) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
5) Wikipedia: Diisobutylaluminium hydride (link)
6) www.sigmaaldrich.com: Diisobutylaluminum hydride solution - 1.0 M in methylene chloride (link)
