THP Protection
(p-TsOH)
Examples:
Example 1

A mixture of the SM (4.1 g, 20 mmol), 3,4-dihydro-2H-pyran (2.0 g, 24 mmol), and p-TsOH monohydrate (100 mg, 0.52 mmol) in anhydrous DCM (70 mL) was stirred at RT for 16 h. The mixture was diluted with DCM and washed with sat aq NaHCO3, then brine. The org layer was separated, dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by silica gel chromatography (0-10% EtOAc/hexane) to provide the product as a clear oil. [4.36 g, 75%]
[Patent Reference: WO2005082859, page 296, ![]() (25.9 MB)]
(25.9 MB)]
Example 2

A mixture of the SM (17.8 g, 55.6 mmol), 3,4-dihydro-2H-pyran (233 g, 2.77 mol), and p-TsOH monohydrate (2.1 g, 11 mmol) in THF (800 mL) was stirred at reflux for 18 h. TEA (10 mL, 72 mmol) was added, and the mixture was concentrated in vacuo. The resulting material was purified by silica gel chromatography (0-25% EtOAc/petroleum ether) to provide the product as a solid, presumed to be a mixture of diastereomeric atropisomers from its 1H NMR. [20 g, 88%]
[Patent Reference: WO2015162516, page 99, ![]() (5.9 MB)]
(5.9 MB)]
Example 3
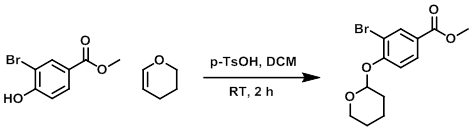
To a stirred solution of the SM (38 g, 164 mmol) and 3,4-dihydro-2H-pyran (45 mL, 493 mmol) in DCM (355 mL) was added p-TsOH hydrate (0.63 g, 3.30 mmol). The reaction was stirred at RT for 2 h (monitored by TLC). The reaction mixture was washed with a mixture of sat aq NaHCO3/brine/H2O (1:1:2). The aq layer was extracted with ether (3x). The combined organics were dried (Na2SO4) and concentrated. The resulting material was purified on silica gel (0-10% EtOAc/hexane) to give a white solid. The solid was recrystallized from MeOH to provide the product. [90%]
[Patent Reference: WO2010045258, page 120, ![]() (12.0 MB)]
(12.0 MB)]
Example 4
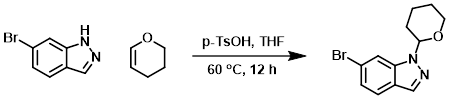
To a mixture of the SM (750 mg, 3.83 mmol) in THF (15 mL) was added 3,4-dihydro-2H-pyran (2.8 g, 7.66 mmol) and p-TsOH (132 mg, 0.77 mmol). The reaction mixture was stirred at 60 C for 12 h, after which time it was concentrated in vacuo. The residue was diluted with EtOAc (50 mL) and washed with H2O (3 x 10 mL). The org layer was dried (Na2SO4) and concentrated. The resulting material was purified by silica gel column chromatography (10:1 PE/EtOAc) to provide the product as a yellow solid. [783 mg, 73%]
[Patent Reference: WO2016011390, page 217, ![]() (20.2 MB)]
(20.2 MB)]
