Sonogashira
Examples:
Example 1
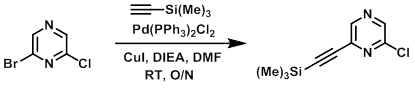
A mixture of the bromide (0.976 g, 5 mmol), Pd(PPh3)2Cl2 (0.106 g, 0.15 mmol), DIEA (0.646 g, 5 mmol), and CuI (0.095 g, 0.5 mmol) in DMF was purged with nitrogen and treated with the alkyne (0.491 g, 5 mmol). The resultant mixture was stirred at RT overnight. The reaction mixture was poured into sat aq NaHCO3 and extracted with EtOAC. The organic layer was washed with brine, dried (Na2SO4), decanted, concentrated, and purified by flash chromatography (0-20% EtOAc/hexane) to provide the product as a clear oil. [861 mg, 80%]
[Patent Reference: WO2010016005, page 86, ![]() (11.3 MB)]
(11.3 MB)]
Example 2
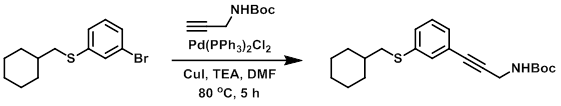
A solution of the bromide (0.508 g, 1.78 mmol), the alkyne (0.414 g, 2.67 mmol), TEA (5 mL), and dry DMF was degassed by bubbling argon for 2 min. CuI (0.010 g, 0.053 mmol) and Pd(PPh3)2Cl2 (0.040 g, 0.057 mmol) were added and the mixture was again degassed by bubbling argon. The mixture was then purged by applying vacuum, then filling with argon (repeated total of 3X). The rxn was stirred under argon at 80 C for 5 h. The mixture was concentrated in vacuo and purified by flash chromatography (5-30% EtOAc/hexane) to provide the product as a light yellow oil. [0.273 g, 43%] [UK Pat App GB2463151A, page 118]
