SNAr (Br)
(Aromatic Alcohols)
Examples:
Example 1
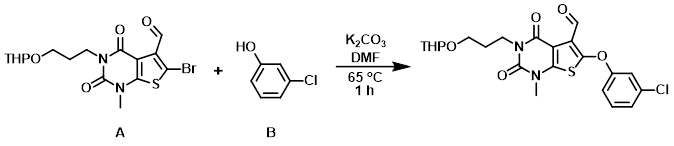
To a mixture of the aryl bromide (A) (100 mg, 0.23 mmol), K2CO3 (159 mg, 1.15 mmol), and DMF (3 mL) was added the phenol (B) (90 mg, 0.70 mmol). The reaction mixture was stirred at 65 C for 1 h, after which time it was diluted with EtOAc (30 mL) and H2O (30 mL). The org layer was dried (Na2SO4) and concentrated. The resulting residue was purified by chromatography (5:1 PE/EtOAc) to provide the product as a yellow oil. [65 mg, 59%]
[Patent Reference: WO2016023832, page 44, ![]() (3.2 MB)]
(3.2 MB)]
Example 2
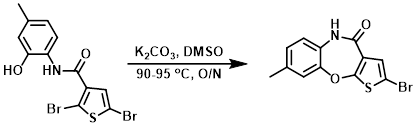
A reactor was charged with the SM (200 g, 511.40 mmol), followed by DMSO (4.00 L), with stirring at ambient temp until dissolved. K2CO3 (84.81 g, 613.68 mmol) was added and the reaction mixture was stirred overnight with a heat jacket set to 90 C. The temp was raised to 95 C for 1 h. The jacket temp was then set to 40 C and the mixture was diluted with H2O (4.00 L) at a rate such that the internal temp did not rise above 55 C. The mixture was stirred for 30 min and then filtered into two equal portions through large sintered funnels. Each bed was washed with H2O (1 x 1.50 L), MeOH (2 x 1.00 L), and finally diethyl ether (1 x 1.00 L), and then thoroughly dried. The two batches were combined and ground up with a mortar and pestle to give a free flowing grey powder that was dried to a constant weight in vacuo over P2O5 to provide the product. [114.6 g, 72.3%]
[Patent Reference: WO2015167890, page 9, ![]() (1.6 MB)]
(1.6 MB)]
