Ester to Alcohol
(LiAlH4)
Examples:
Example 1
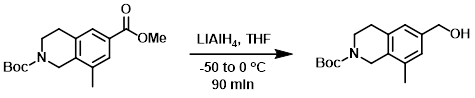
To a solution of the SM (21 g, 69.3 mmol) in dry THF (500 mL) at -50 C was added LiAlH4 (7.4 g, 208 mmol). The reaction mixture was stirred at -50 C for 1 h, then 0 C for 30 min. The mixture was slowly quenched with H2O (7.4 mL), followed by 15% aq NaOH (7.4 mL), then additional H2O (22.2 mL). The mixture was filtered and the filtrate was concentrated in vacuo. The residue was purified by Prep HPLC and the resulting eluent was concentrated to remove volatile organics. The remaining aq mixture was extracted with DCM. The combined organics were dried (Na2SO4) and concentrated in vacuo to provide to product as a colorless oil. [14.8 g, 77%]
[Patent Reference: WO2016014463, page 95, ![]() (6.7 MB)]
(6.7 MB)]
Example 2

The SM (900 mg, 4.1 mmol) in dry THF (6.5 mL) was added dropwise to a suspension of LiAlH4 (188 mg, 4.9 mmol) in dry THF (6.5 mL) at 0 C under N2. The mixture was stirred for 30 min, after which time it was very slowly quenched with H2O (1 mL) and diluted with DCM. The mixture was stirred 20 min. The mixture was filtered through a celite pad and the filtrate was dried (MgSO4) and concentrated to provide the product as a colorless oil. [845 mg, 100%]
[Patent Reference: WO2015144799, page 106, ![]() (18.8 MB)]
(18.8 MB)]
Example 3

To a suspension of LiAlH4 (1.17 g, 30.8 mmol) in diethyl ether (70 mL) at -45 C was added a solution of the SM (2.30 g, 14 mmol) in diethyl ether (14 mL). The resulting mixture was stirred at -45 C for 1h, warmed to -10 C for 1 h, then slowly warmed to RT and stirred for 15 h. The reaction mixture was cooled to 4 C and treated sequentially with H2O (1.2 mL), 10% aq NaOH (1.2 mL), and H2O (3.6 mL). Diethyl ether (50 mL) was then added and the solution was allowed to stir for 90 min. The organic layer was separated and the aq phase back extracted with diethyl ether (3 x 50 mL). The combined org layers were dried (MgSO4), filtered, and concentrated in vacuo to give a 45% solution of the title compound in diethyl ether. [1.56 g, 92%]
[Patent Reference: WO2010032200, page 147, ![]() (6.2 MB)]
(6.2 MB)]
Example 4
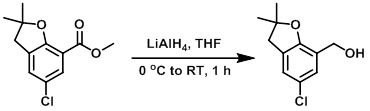
To a solution of the SM (2.00 g, 8.31 mmol) in dry THF (15 mL) at 0 C under N2 was added LiAlH4 (1.0 M in THF, 8.31 mL, 8.31 mmol) over 10 min. The reaction mixture was allowed to warm to RT and stir for 1 h. The mixture was cooled to 0 C and quenched by the addition of EtOAc (10 mL) followed by sat aq Na2SO4 (10 mL). The mixture was diluted with EtOAc and filtered through a pad of celite. The filtrate was dried (Na2SO4), concentrated, and purified by silica gel chromatography (0-100% EtOAc/hexane) to provide the product.
[Patent Reference: WO2011159297, page 82, ![]() (10.6 MB)]
(10.6 MB)]
Example 5

To a solution of the SM (20.0 g, 67.7 mmol) in THF (200 mL) at 0 C was added LiAlH4 (5.6 g, 203.4 mmol) slowly over 1 h. The reaction mixture was stirred at RT for 3 h. Upon completion, the mixture was quenched with sat aq Na2SO4. The solids were removed by filtration and the filtrate was extracted with EtOAc. The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a colorless liquid. [18.0 g, 99%]
[Patent Reference: WO2014149164, page 233, ![]() (23.7 MB)]
(23.7 MB)]
Example 6

To a suspension of LiAlH4 (5.7 g, 150 mmol) in THF (150 mL) at 0 C under N2 was added dropwise a solution of the SM (15 g, 75 mmol) in THF (50 mL). The reaction was allowed to warm to RT and stir for 3 h. The mixture was quenched with 10% NaOH (5.7 mL), then H2O (5.7 mL), after which time it was filtered to remove solids. The filtrate was diluted with H2O and extracted with EtOAc (3x). The combined organics were washed with brine, dried (MgSO4), and concentrated to provide the product. [10 g, 84%]
[Patent Reference: WO2013134298, page 40, ![]() (4.1 MB)]
(4.1 MB)]
Example 7
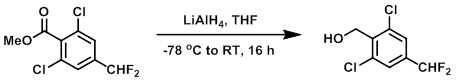
To a solution of the SM (1.44 g, 5.64 mmol) in THF (10 mL) at -78 C was added dropwise LiAlH4 (2.0M in THF, 4.23 mL, 8.46 mmol) in THF (10 mL) over 15 min. The reaction mixture was stirred at 0 C for 30 min, then RT for 16 h. The mixture was quenched with 1M HCl (20 mL) at 0 C, stirred 20 min, then extracted with EtOAc (2 x 30 mL). The combined organics were washed with H2O (50 mL), brine (50 mL), dried (Na2SO4), and concentrated to provide the product as a colorless oil. [0.59 g, 45%]
[Patent Reference: WO2015129926, page 96, ![]() (21.5 MB)]
(21.5 MB)]
Example 8
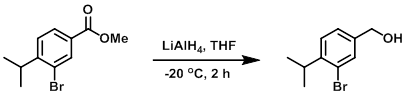
LiAlH4 (5.52 g, 145 mmol) was added to a stirred solution of the SM (34.0 g, 132 mmol) in THF (600 mL) at -20 C. The reaction mixture was stirred at -20 C for 2h. The mixture was quenched with H2O (5.26 mL), 3N NaOH (5.52 mL), and H2O (16 mL). The solids were removed by filtration and washed with DCM. The filtrate was concentrated in vacuo to provide the product as a yellow oil. [20.0 g, 66%]
[Patent Reference: WO2015144799, page 176, ![]() (18.8 MB)]
(18.8 MB)]
Example 9

To a suspension of LiAlH4 (1.71 g, 45.0 mmol) in anhydrous THF (80 mL) was added the SM (6.6 g, 22.5 mmol) dropwise at 0 C. The reaction mixture was warmed to RT and stirred for 12 h. The mixture was quenched with H2O and diluted with EtOAc (50 mL). The mixture was washed with H2O (2 x 50 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel chromatography (10-30% EtOAc/hexane) to provide the product as a colorless oil. [2.2 g, 47%]
[Patent Reference: WO2015140133, page 104, ![]() (11.7 MB)]
(11.7 MB)]
