THP Protection
(Pyridinium p-TsOH)
Examples:
Example 1
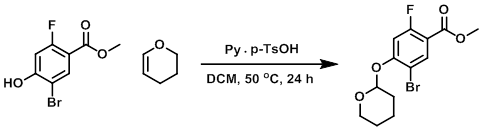
To a flask containing the SM (13.15 g, 52.8 mmol) in dry DCM (90 mL) was added 3,4-dihydro-2H-pyran (10 mL, 110 mmol) followed by pyridinium p-TsOH (0.13 g, 0.53 mmol). The reaction mixture was stirred at a gentle reflux (50 C) for 24 h (monitored by TLC and LCMS). The mixture was concentrated in vacuo. The resulting material was diluted with MeOH and heated in a flask on a rotovap (without vacuum) at 40 C for about 30 min. The mixture was concentrated to about 5 mL in volume, cooled to RT, and filtered. The resulting white solid was rinsed with MeOH (1x) to provide the product. [13.35 g, 76%]
[Patent Reference: WO2010045258, page 141, ![]() (12.0 MB)]
(12.0 MB)]
Example 2
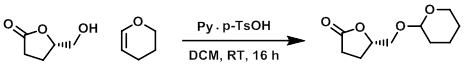
To a solution of the SM (4.0 g, 34.45 mmol) in DCM (20 mL) was added 3,4-dihydro-2H-pyran (3.95 mL, 41.34 mmol) followed by pyridinium p-TsOH (0.86 g, 3.44 mmol) at RT and the reaction mixture was stirred for 16 h. The mixture was diluted with DCM (20 mL), quenched with H2O (40 mL), and extracted with DCM (2 x 50 mL). The combined organics were washed with brine (2 x 20 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (50% EtOAc/hexane) to provide the product as a colorless gum. [5.85 g, 85%]
[Patent Reference: WO2015129926, page 88, ![]() (21.5 MB)]
(21.5 MB)]
Example 3
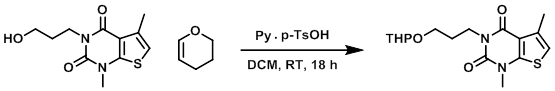
To a solution of the SM (9 g, 35.4 mmol) and pyridinium p-TsOH (900 mg, 3.54 mmol) in DCM (90 mL) at 0 C was added 3,4-dihydro-2H-pyran (8.96 g, 106 mmol) dropwise. The reaction mixture was stirred at RT for 18 h. The mixture was poured into H2O (30 mL) and extracted with DCM (2 x 20 mL). The combined organics were washed with brine (20 mL), dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by chromatography (4:1 PE/EtOAc) to provide the product as a yellow oil. [11.2 g, 93.4%]
[Patent Reference: WO2016023832, page 42, ![]() (3.2 MB)]
(3.2 MB)]
