TBS Deprotection
(Cs2CO3)
Examples:
Example 1
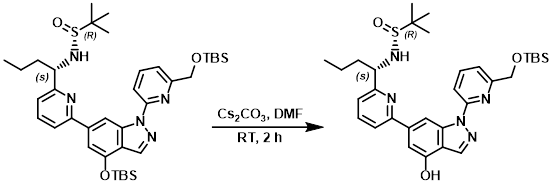
A mixture of the SM (800 mg, 1.11 mmol) and Cs2CO3 (721 mg, 2.22 mmol) in DMF (10 mL) was stirred at RT for 2 h. The mixture was diluted with H2O (60 mL) and extracted with EtOAc (90 mL). The org phase was washed with brine, dried (Na2SO4), and concentrated. The residue was purified by silica gel chromatography (1:2 PE/EtOAc) to provide the product as a yellow solid. [600 mg, 89%]
[Patent Reference: WO2016011390, page 274, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
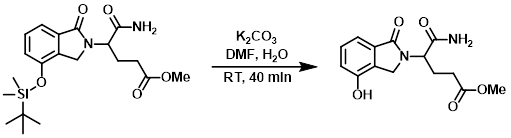
To a stirred, cold solution of the SM (152 g, 288 mmol) in DMF (500 mL) and H2O (55 mL) was added K2CO3 (19.89 g, 144 mmol) in portions over 5 min. The reaction mixture was stirred at RT for 40 min, after which time it was cooled in an ice bath. To the mixture was slowly added HCl (12M, 23.99 mL, 288 mmol). After the addition, ACN (280 mL) was added to the mixture and a solid precipitated out. The mixture was stirred at RT for 10 min and filtered. The solid was washed with ACN (4 x 50 mL). The filtrate was concentrated in vacuo to give a yellow oil (168 g). The oil was dissolved in ACN (600 mL) and stirred at RT for 10 min. The mixture was filtered and the solid was washed with ACN (2 x 25 mL). The filtrate was concentrated to give a yellow oil (169 g), which was added to a mixture of H2O (1.2 L) and ether (1.0 L). The mixture was stirred for 3 min and the layers separated. The aq solution was concentrated in vacuo and the residue was stirred in ACN (160 mL), and a white solid formed after stirring overnight. The mixture was filtered to provide the product as a white solid (46 g, 54%). The filtrate was concentrated and the residue was further crystallized in ACN (60 mL) to provide more product as a white solid (11.7 g, 14%). The filtrate was concentrated and the residue purified by ISCO chromatography to provide additional product as a white solid (13.2 g, 15%). [Total Yield: 70.9 g, 83%]
[Patent Reference: WO2014025960, page 97, ![]() (8.1 MB)]
(8.1 MB)]
