Acid to Ester
(SOCl2/MeOH)
Examples:
Example 1

To a solution of the SM (1 g, 3.7 mmol) in MeOH (15 mL) was slowly added SOCl2 (5 mL) at 0 C. The reaction mixture was refluxed at 70 C for 2 h, after which time it was cooled to RT. MeOH was removed in vacuo and the resulting residue was poured onto ice-H2O (25 mL) and extracted with EtOAc (2 x 10 mL). The combined org extracts were washed with 10% NaHCO3 (2 x 10 mL), brine, dried (Na2SO4), and concentrated to provide the product as a yellow solid. [900 mg]
[Patent Reference: WO2010038081, page 128, ![]() (33.8 MB)]
(33.8 MB)]
Example 2

To a solution of the SM (15.0 g, 88.0 mmol) in MeOH (90.0 mL) at 0 C was added dropwise SOCl2 (13.0 mL). The resulting mixture was refluxed for 12 h, after which time it was concentrated in vacuo. The resulting material was diluted with H2O (150 mL) and extracted with EtOAc. The org layer was washed with aq NaHCO3 (50 mL), brine (50 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as an off-white solid. [15.0 g, 92.5%]
[Patent Reference: WO2014149164, page 259, ![]() (23.7 MB)]
(23.7 MB)]
Example 3
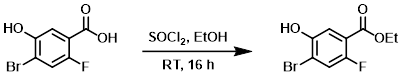
To a solution of the SM (25 g, 106.4 mmol) in EtOH (250 mL) at 0 C was added dropwise SOCl2 (15.5 mL, 212.8 mmol). The reaction mixture was stirred at RT for 16 h, after which time the solvent was removed in vacuo. To the residue was added EtOAc (250 mL) and the mixture was washed with sat aq NaHCO3 (2 x 100 mL). The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as an off-white solid. [11 g, 39%]
[Patent Reference: WO2015051043, page 98, ![]() (9.7 MB)]
(9.7 MB)]
